|
x |
x |
|
 |
 |
|
INFECTIOUS
DISEASE |
BACTERIOLOGY |
IMMUNOLOGY |
MYCOLOGY |
PARASITOLOGY |
VIROLOGY |
|
En
Español |
VIROLOGY -
CHAPTER SIXTEEN
PARAINFLUENZA, RESPIRATORY
SYNCYTIAL AND ADENO VIRUSES Dr Margaret
Hunt
Professor Emerita
University of South Carolina School of Medicine
|
|
TURKISH |
|
SHQIP - ALBANIAN |
Let us know what you think
FEEDBACK |
|
SEARCH |
|
|

 |
|
Logo image © Jeffrey
Nelson, Rush University, Chicago, Illinois and
The MicrobeLibrary |
|
|
|
|
|
TEACHING OBJECTIVES
Brief review of paramyxovirus virus structure, properties and
classification.
Discussion of human parainfluenza virus infections, disease,
epidemiology, prevention and treatment.
Discussion of respiratory syncytial virus infections, disease,
epidemiology, prevention and treatment.
Discussion of human metapneumovirus infections, disease, epidemiology,
prevention and treatment.
Brief review of adenovirus structure, properties and classification
Discussion of adenovirus infections, disease, epidemiology, prevention
and treatment.
|
The common cold
The common cold is as an acute, self-limited catarrhal (Latin
catarrhus, to flow down) syndrome limited to the mucosal membranes
of the upper respiratory tract. Rhinoviruses, of which there are more
than 100 serotypes, cause an estimated 30% to 50% of colds.
Coronaviruses account for perhaps 10% of cases.
The viruses covered in this chapter are additional frequent causes of
the common cold, However, they can also cause more serious disease.
PARAMYXOVIRUSES
GENERAL
Classification
Family Paramyxoviridae
Genus Members
Paramyxovirus
-
Parainfluenza [PIV types 1,2,3,4]
Rubulavirus - Mumps virus
Newcastle Disease Virus [birds]
Sendai virus [mice]
Morbillivirus
- Measles
virus
Canine Distemper Virus
Pneumovirus
-
Respiratory Syncytial Virus (RSV)
Metapneumovirus
Structure
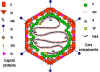
Paramyxoviruses are enveloped RNA viruses.
Their RNA is negative-sense and is non-segmented. The nucleocapsid
has helical symmetry
The vriral envelope
contains two virally coded glycoproteins (table 1):
-
The F protein
which has fusion activity.
-
The attachment protein - H, HN or G
according to whether is has hemagglutination activity (H),
hemagglutination plus neuraminidase activity (HN) or neither (G)
The general structure of paramyxovirsues is
shown in figure 1
|
Table 1
Paramyxovirus family surface glycoproteins |
| GENUS |
GLYCOPROTEINS |
TYPICAL MEMBERS |
| PARAMYXOVIRUS
SUB-FAMILY |
| Paramyxovirus |
HN, F |
HPIV1, HP1V3 |
| Rubulavirus |
HN, F |
HPIV2, HPIV4, Mumps virus |
| Morbillivirus |
H, F |
Measles virus |
| PNEUMOVIRUS
SUB-FAMILY |
| Pneumovirus |
G, F |
Respiratory syncytial virus |
| Metapneumovirus |
G, F |
Metapneumoviruses |
PARAINFLUENZA VIRUS
Parainfluenza viruses are important viral pathogens causing
upper and lower respiratory infections in adults and children. They are
second to respiratory syncytial virus as a cause of lower respiratory tract
disease in young children.
|
|
|
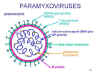 Figure 1. Structure of a paramyxovirus
Figure 1. Structure of a paramyxovirus
 Figure 2. Paramyxovirus
Figure 2. Paramyxovirus
© Dr Linda Stannard, University of Cape Town, South Africa (used with permission)
|
Structure
Parainfluenza viruses are relatively large
viruses of about 150-300 nm in diameter. They have a
spherical or pleomorphic shape (figure 1 and 2). The RNA is negative sense,
unsegmented and single stranded (ss). The nucleocapsid core is filamentous
or herringbone-like, has helical RNA tightly associated with
Nucleoprotein (NP) Phosphoprotein (P) and Large protein (L)
These are enveloped viruses with a
host-derived lipid bilayer associated with two virus-specific
glycoproteins:
Hemagglutinin-Neuraminidase
(HN). This is
a viral attachment protein, that also causes hemadsorption and
hemagglutination.
Fusion protein
(F). The
F protein forms
spikes out from the envelope. It promotes the fusion of host and
viral cell membranes which is an initial step in infection. It is
synthesized as a biologically inactive form (F0), which
is activated by proteolytic cleavage to an active form that has 2
subunits, F1 and F2, linked by a disulfide
bond.
Matrix (M) protein, located just
within the envelope, is hydrophobic
|
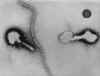 Figure 3.
Figure 3.
Transmission electron micrograph of parainfluenza virus. Two intact particles and free filamentous
nucleocapsid.
CDC/Dr. Erskine Palmer |
|
Table 2. Proteins of Parainfluenza Virus |
|
Structural
Protein |
Designation |
Location |
Function |
|
Hemagglutinin-neuraminidase
(glycoprotein) |
HN |
Envelope |
Attachment to host cell
receptors, hemagglutinin and neuraminidase activity
|
|
Fusion Protein
Matrix Protein
Nucleoprotein
Phosphoprotein
Large Protein
|
F
M
NP
P
L |
Envelope
Inside the envelope
Nucleocapsid
Nucleocapsid
Nucleocapsid |
Fusion, penetration,
hemolysis
Assembly
Complexed with RNA genome,
Part of the RNA polymerase
complex
Part of the RNA polymerase
complex |
|
| |
Isolation
Cell lines such as primary Rhesus
monkey kidney epithelial Cells (PRMK), LLC-MK-2, and human embryonic
kidney cells are used. Cytopathic effects occur such as rounding,
bridging, cell lysis, and syncytium formation.
Hemadsorption (due to the
interaction of viral hemagglutinin with specific erythrocyte receptors on
guinea pig red cells) can be observed at 4° C. This may be seen even
before the appearance of cytopathic effects and has been used for early
diagnosis (especially PIV-1 and PIV-3).
Pathogenesis
The first step in the infection
cycle involves attachment of the virus to host cell sialic acid receptors.
This is mediated by viral attachment protein, a function served by the HN
glycoprotein.
Next, the F protein catalyzes fusion
of the viral envelope and host cell membrane, resulting in uncoating and
release of the nucleocapsid structure into the host cell cytoplasm.
For transcription and protein
synthesis to occur, first mRNA is formed with the help of RNA-dependent
RNA polymerase which must be supplied by the virus. The polymerase function is carried out by the P and L
proteins, and possibly also the NP. The genome is replicated by formation
of a full-length positive sense RNA template from which a negative sense
RNA is then transcribed.
Assembly of the nucleocapsid occurs
and M proteins are then associated with the viral glycoprotein modified
cell membranes. Mature virions are released from host cell membranes by
budding.
|
| |
Epidemiology and
Transmission
The virus is ubiquitous,
restricted to humans and antigenically stable.
Most
people have had a primary infection by all four serotypes by the age of five;
infections occur as epidemics as well as sporadically. There can be
repeated infections throughout life.
People usually shed virus for about 1 week but immunocompromised
individuals may shed for much longer.
Parainfluenza viruses are sensitive to detergents and
heat but can remain viable on surfaces for up to 10 hours.
Transmission occurs via the
following routes:
- Large droplets - person to person
through close contact
- Aerosols of respiratory secretions
- Fomites (virus survives on
surfaces): The virus is relatively unstable, but can survive on
surfaces for a few hours.
Parainfluenza virus is highly contagious.
|

Weekly reports of parainfluenza type 1 in the US.
Seasonal variation. CDC

Weekly reports
of parainfluenza type 2 in the US. Seasonal variation. CDC
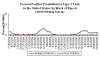
Weekly reports
of parainfluenza type 3 in the US. Seasonal variation. CDC
|
Clinical Features
Primary infections and re-infections
occur but most infections are asymptomatic,
especially in older children and adults. The incubation period is 2 to 6 days. Reinfections are clinically less
severe, most commonly involve the upper respiratory tract and occur
throughout life.
Fever and a spectrum of respiratory
infections are caused by PIVs:
- Rhinorrhea/rhinitis,
pharyngitis,
cough, croup (laryngotracheobronchitis),
bronchiolitis, and pneumonia.
- Croup - the subglottic region becomes
narrower and results in difficulty with breathing, a seal bark-like cough
and hoarseness. It is associated with fever, cough, hoarseness,
stridor on inspiration and expiration.This
is predominantly a disease of children under six years
of age (because they have narrower airways). Many viruses can cause
croup but HPIV is the most common cause (HPIV1>HPIV2>HPIV3).
HPIV types 1 and 2 most often cause
outbreaks of croup in autumn/early winter, with an alternate year pattern.
PIV-1 tends to attack children ages 2-6 years.
HPIV-3 can cause croup, though less
commonly than PIV-1 and 2 and is sporadic. It often occurs in the spring and summer. Primary infection with PIV 3 in
young infants and children of less than two years of age is a common cause of
bronchiolitis (although RSV is a more common cause).
HPIV-4 is associated with mild upper
respiratory infections. The upper airway is more often involved than the
lower airway. There are two types of PIV4: A and B
Otitis
media,
parotitis, aseptic
meningitis occur although they are rare.
Particularly severe and persistent
infections are known to occur in immunocompromized children and adults
in whom
prolonged viral shedding is seen.
|
| |
Clinical Diagnosis
Antigen detection
Radio-immunoasay, enzyme
immunoassay, fluoro-immunoassay, and immunofluoresence methods are used for antigen
detection. Nasopharyngeal secretions are
collected, from swabs or washings, and transported in viral transport
medium and on ice. These techniques are 70-90% sensitive.
Shell vial assay is useful in
detecting growth in 4-7 days. Hemadsorption can be noted before
cytopathic effects.
Immunofluoresence is confirmatory.
Antibody Detection
Serology uses hemagglutinin
inhibition to demonstrate a difference between acute and convalescent
levels. A 4-fold increase in antibody titers is considered positive.
However, serologic diagnosis is of limited value because of the presence
of nonspecific inhibitors and the antibody being heterotypic (antibody that is common to different PIV types as well as the mumps
virus)
Treatment
Most HPIV infections are mild and self-limiting.
There is no specific treatment and no
anti-virals are available. Supportive treatment for croup
includes humidification of air and racemic epinephrine (Racemic
epinephrine is a 1:1 mixture of the dextrorotatory and levorotatory
isomers of epinephrine. The L form is the active component) . Corticosteroids
may be used in moderate to severe cases.
Immunity
Immunity following infection is
short lived. The role of antibody is not clear since reinfection has been seen even with high levels of antibody. Cell-mediated
immunity is
probably more important for limiting infection.
Breastfeeding may protect babies from HPIVs during their first few months
of life because mothers may have protective antibodies in their breast milk.
Infection control
Asymptomatic shedding is common,
making it difficult to contain spread of infection. Hand washing and
preventing contamination of surfaces with respiratory secretions are
important for limiting nosocomial spread.
|
|
|
RESPIRATORY
SYNCYTIAL VIRUS
Classification and
structure
Family Paramyxoviridae, genus
Pneumovirus.
Infection of cells by RSV often results in syncytium
formation,
hence the name. The virus was first discovered in chimpanzees (Chimpanzee
Coryza Virus) and accidental infection in humans led to its recognition as a
human pathogen.
These are spherical or pleomorphic enveloped
viruses (100-350 nm) with single-stranded, negative sense linear RNA.
There are two non-structural and eight structural proteins.
The envelope has two glycoproteins:
- F
protein, the
fusion
protein, is important for fusion of viral particles to target cells and
fusing infected cells to neighboring cells to form syncytia.
- G protein,
which is highly glycosylated, is important for viral attachment to host cells. Antigenic variations in the type of G
protein determine the subgroup (A or B).
RSV lacks H/N proteins, unlike
other members of the family Paramyxoviridae.
Properties
These viruses survive on surfaces for
up to six
hours, on gloves for less than two hours. They rapidly lose viability with freeze-thaw
cycles, in acidic conditions and with disinfectants.
Pathology and Pathogenesis
RSV attaches (via the G protein) to
cells of nasal mucosa and upper respiratory tract. The F protein allows
fusion of the viral envelope with the host cell plasma membrane. The
virus can also infect the eye. Infected cells may undergo necrosis and syncytia form through
cell-cell fusion (which is often seen with cultured cells). Cell to cell transfer of virus leads
to spread from upper to lower respiratory tract.
Mucosal edema occurs and there is increased mucin secretion. There is
also cell necrosis that leads to sloughing of debris.
Smaller airways (bronchioles) become
plugged with debris and mucin; bronchoconstriction also occurs.
Peribronchial lymphocytes may infiltrate the tissue.
The host
immune response also induces some of the pathological changes. IgE
response in some individuals is linked to airway hyper-reactivity.
Cell-mediated immunity and humoral response limits the severity of the
infection.
Epidemiology
RSV has a worldwide distribution and
and is an important cause of lower respiratory tract disease in young
infants. Most children have had an RSV infection
by age 4 years. RSV is the most frequent cause of bronchiolitis but is an
infrequent cause of croup.
Outbreaks are seasonal occurring
from late fall through
spring (November to May), the virus being transmitted via large droplets,
through
fomites and via
the hands. RSV can survive on surfaces for up to six hours. Viral shedding continues for
less than 1
to 3 weeks but longer in immuno-compromised hosts.
Nosocomial spread is common. Viral
shedding can last for up to three weeks and infants can show a high titer of
shed virus, especially initially (107 viral particles per ml).
Asymptomatic viral shedding is also observed. There is prolonged shedding in
immunocompromized individuals.
75,000 to 125,000 infants are hospitalized each year in the US because of
RSV infections. These account for 50 to 90% of hospitalizations for
bronchiolitis.
|
 Figure .
Figure .
Transmission electron micrograph of respiratory syncytial virus. Long
filamentous form.
CDC/Dr. Erskine Palmer
Morphologic traits of the Respiratory Syncytial Virus. The virion is
variable in shape, and size (average diameter of between 120-300nm)
|
|
WEB RESOURCES
CDC
RSV information
RSV
in a child-care situation (CDC) |
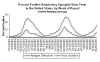
Weekly reports of RSV isolation in the US

Section of lung: acute pneumonia, epithelial syncytia formation in alveoli, respiratory syncytial virus
infection, calf pneumonia
© Bristol Biomedical Image Archive. Used with permission
|
Clinical Features
The incubation period is 4 to 6 days
(range: 2 to 8 days). First, there is an upper respiratory infection (‘bad
cold’) in older children and adults with clinical features of fever,
rhinitis, pharyngitis. Lower respiratory infection (bronchiolitis and/or pneumonia) may occur after the upper
respiratory infection and results in the clinical features of cough,
tachypnea,
respiratory distress,
hypoxemia, cyanosis.
The cough can persist for 3 weeks.
In young infants one may observe
apnea, lethargy,
irritability, poor feeding, otitis media and croup.
Radiological examination may show
atelectasis, streaking, hyperinflation
and perihilar infiltrates, especially in the right middle and upper
lobes.
Severe infections occur in
pre-term
infants (especially less than 35 weeks gestation and those with chronic lung
disease), children with cyanotic congenital heart disease, and
immunocompromized hosts. There are up to 3000 deaths per year in the
United States.
|
| |
Diagnosis
Nasal washings, nasal aspirates or
swabs should be transported on ice and processed immediately. Rapid diagnosis
is carried out using DFA, IFA, ELISA.
Viral culture is carried out in cell lines such as
HeLa, Hep-2, Monkey Kidney cells. Cytopathic effects are usually seen
in 2-5 days. Shell vial technique with immunofluorescence is useful.
Treatment
Treatment is usually supportive by
the provision of fluids, oxygen,
humidification of air, respiratory support. Steroids and bronchodilators
have not proved useful.
Chemotherapy
Ribavirin (Virazole) (see
chemotherapy) , a guanosine analogue
(aerosol) has been used with some efficacy but is used only in persons
at high risk for severe disease (premature and immunocompromized infants).
An experimental nucleoside-analog drug taken orally, ALS-008176, has
shown promise in decreasing viral load and increasing clearance. The
time until the virus was undetectable in the experimental protocol was
1.3 to 2.3 days, depending on the dosage, compared to 7.2 days for
patients given a placebo. This drug appears to inhibit replication of
RSV in already infected cells as well as protecting epithelial cells.
Immunity
Humoral immunity
Neutralizing antibodies are
against F and G proteins. IgA is also produced during an infection. The level of neutralizing antibody does
not correspond to neutralizing activity. Immunity is short lived, therefore reinfections are common. Newborns may have some innate
immunity
An IgE response occurs in some
individuals and may be a marker for future airway hyper-reactivity.
Cell mediated immunity
This is carried out by T cells. Cytokine
production contributes to illness.
|
| |
Prevention of
spread
Hand washing is important as is isolation and cohort nursing.
Health care providers should wear protective gear, i.e. gowns, gloves,
masks and goggles
Immunization
Vaccine
The inactivated
vaccine is no longer used because it was associated with an increase in
severity of disease. Other subunit vaccine candidates are in trial phases
but no vaccine is available yet.
Passive immunity by Palivizumab
Palivizumab (Synagis) is a humanized monoclonal antibody that gives
passive immunity against RSV. It is made by recombinant DNA technology.
The constituent antibodies bind an epitope in the A antigenic site of
the RSV envelope fusion (F) protein on the surface of the virus thus
blocking membrane fusion. It also prevents cell-cell fusion of
RSV-infected cells. Certain RSV variants are resistant to Palivizumab in
laboratory experiments as a result of mutation in the F protein at the
antibody binding site. No known sequence variations outside the A
antigenic site on RSV F have been demonstrated to render RSV resistant
to neutralization by Palivizumab.
Palivizumab injections are recommended for infants that are high-risk
for serious lower respiratory tract disease caused by RSV because of
prematurity or other medical problems such as congenital heart disease.
In Phase III clinical trials, Palivizumab reduced the risk of
hospitalization as a result of RSV infection by about 50%. It is given
once a month via intramuscular injection during the RSV season. It is
also very expensive.
|
|
|
Negative-stain electron micrographs of human
metapneumovirus.
Photograph courtesy of Dr. Charles Humphrey of CDC/NCID/IDPA
Published in JID 2002;185:1660-3 |
HUMAN
METAPNEUMOVIRUS
This virus (Pneumovirinae subfamily, Paramyxoviridae family)
is closely related to RSV and was first recognized as a pathogen in the
Netherlands in 2001. Its role in upper and lower respiratory tract
infections is now being recognized world-wide and it may cause 5% of
respiratory illness in children. There is often co-infection with RSV.
Metapneumovirus is ubiquitous and, by the age of five, most people are
seropositive and have thus been infected by the virus. Many infections are
asymptomatic but the virus can cause both upper and lower respiratory tract
infections with symptoms of a cold, otitis media, pneumonia or
bronchitis. There can also be pneumonia in marrow recipients that can
possibly be fatal. There are distinct epidemics in the winter months. There are two main HMPV
types (A and B), each with 2 subtypes (A1, A2; B1, B2) and allfour of these
circulate in the population with the dominant strain varying.
Metapneumovirus can be detected by PCR but there is no commercially available
testing. |
| |
ADENOVIRUS |
|
|
These viruses
were named
"adenovirus" because they were first isolated in 1953 from
tissue cultures of human adenoidal tissue.
Classification
Adenoviruses belong to family Adenoviridae,
genus Mastadenovirus.
They are further classified
into 6 subgroups (A through F), based on hemagglutinating properties and
DNA homology.
About 47 serotypes have been
isolated from humans.
Types 40, 41 belong to subgroup F
and are enteric pathogens.
Common serotypes are 1 - 8, 11, 21,
35, 37, and 40.
|
 Structure of adenovirus
Structure of adenovirus
 Adenovirus
© Dr Stephen Fuller, 1998
Adenovirus
© Dr Stephen Fuller, 1998 |
Structure
Adenoviruses are non-enveloped viruses
with a diameter of 70-90nm.
The genome is made of linear
double-stranded (ds) DNA with 2 major proteins.
The capsid is
icosahedral, comprised
of 252
capsomeres. 240 are hexons; at the vertices are 12 pentons, from
which a fiber with a terminal knob projects. This complex is toxic to
cells - causing rounding and death of cells through inhibition of protein
synthesis. The fiber proteins determine target cell specificity.
10 structural proteins are known.
|
 Adenovirus
Adenovirus
© Dr Linda M
Stannard, University of Cape Town, South Africa, 1995 (used with
permission).
Transmission electron micrograph of adenovirus
CDC/Dr. G. William Gary, Jr.
|
Pathogenesis
and Replication
Virus primarily attacks
mucoepithelial cells of the conjunctiva, respiratory tract,
gastrointestinal and genitourinary tracts. Attachment to host cell
receptor occurs via the fiber protein. The virus replicates in the cytoplasm
of host cells, but viral DNA replicates within the host cell nucleus.
Early and late phases of replication occur, followed by assembly and release of
virions.
Three types of infections occur in
target cells:
Lytic - cell death occurs as a
result of virus infection (mucoepithelial cells)
Latent / persistent /
occult
- virus
remains in the host cell, which is not killed (lymphoid tissues such as
tonsils, adenoids, Peyers patches)
Oncogenic transformation - cell
growth and replication continue without cell death. This is seen in hamsters,
most often with group A viruses (see
oncogenic
viruses).
Adenovirus also replicates in
associated lymphoid tissues, and subsequent viremia can cause secondary
infection in visceral organs.
Inefficient (error-prone)
replication of the virus results in many excess antigenic components.
These are liberated into the culture fluid in vitro as soluble
antigens and lead to formation of basophilic staining intra-nuclear
inclusion bodies in cells.
Properties
Adenoviruses are stable in the
environment and to low pH, bile, and proteolytic enzymes - These properties make
it possible for them to replicate to high titers in the GI tract.
|
|
|
| |
Clinical
Syndromes
Almost half of adenoviral
infections are subclinical. Most infections are self-limited and
induce type-specific immunity.
The incubation period is 2-14 days; for
gastroenteritis usually 3-10 days
Different clinical syndromes have
been described:
Eye
Epidemic Keratoconjunctivitis (EKC),
acute follicular conjunctivitis, pharyngoconjunctival fever
Respiratory system
Common cold (rhinitis), pharyngitis
(with or without fever), tonsillitis, bronchitis, pharyngoconjunctival
fever, acute respiratory disease (LRI), pertussis-like syndrome, pneumonia-
sometimes with sequelae
Genitourinary
Acute hemorrhagic cystitis, orchitis,
nephritis, oculogenital syndrome
Gastrointestinal
Gastroenteritis, mesenteric
adenitis,
intussusception, hepatitis, appendicitis.
Diarrhea tends to last longer than
with other viral gastroenteritides
Rare results of adenovirus
infections include: Meningitis, encephalitis,
arthritis, skin rash, myocarditis, pericarditis, hepatitis. Fatal disease
may occur in immunocompromised patients, as a result of a new infection or reactivation of latent virus
|
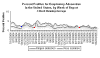
Weekly reports
of respiratory adenovirus in the US. Seasonal variation.
CDC |
|
ADENOVIRUS-
CLINICAL SYNDROMES
|
|
Clinical
Syndrome |
Features |
Serotypes
commonly Involved |
Serotypes
rarely Involved |
|
URI
|
Coryza, pharyngitis,
tonsillitis, fever
|
1, 2, 3, 5, 7
|
4, 6, 11, 18, 21, 29, 30
|
|
Pharyngo-conjunctival fever |
Fever, conjunctivits,
pharyngitis, headache, rash, lymphadenopathy |
3, 4, 7, 14 |
1, 11, 16, 19, 37 |
|
LRI |
Bronchitis, pneumonia, fever,
cough
|
3, 4, 7, 21 |
14, 1, 2, 5, 35
|
|
Pneumonia |
Fever, respiratory distress, cough, severe in young
children and infants
|
7 |
1, 2, 3,4, 14, 21, 7b
|
|
Pertussis-like
Syndrome
|
Fever, paroxysmal cough, post-tussive
vomiting |
5 |
1, 2, 3, 12, 14, 19, 21, 35 |
| Acute Respiratory Disease |
Tracheobronchitis, pneumonia,
fever; epidemics in military recruits |
4, 7 |
2, 3, 5, 8, 11, 14, 21 |
| Epidemic Keratoconjunctivitis |
Headache, conjunctivitis
followed by keratitis, preauricular lymphnodes
|
8, 19, 37 |
2-7, 14, 15, 19, 37 |
|
Acute follicular/
Hemorrhagic conjunctivitis
|
Chemosis, follicles,
subconjunctival hemorrhage, preauricular lymph
nodes |
11 |
|
| Acute Hemorrhagic cystitis |
Blood in urine
(macroscopic hematuria)
fever, dysuria
|
11, 4, 7, 1, 21 |
34, 35 |
| Gastro-enteritis |
Diarrhea especially in
children <4 years old
Low grade fever |
40-42, 31, 25-28, |
3, 7, 2, 9, 12, 13, 18 |
Epidemiology
Endemic, epidemic and sporadic
infections occur. Outbreaks have been noted in military recruits, swimming pool
users, residential institutions, hospitals, day care centers etc.
Transmission is by droplets, the
fecal-oral route (direct and through poorly chlorinated water) or fomites
Many infections are subclinical
Infections are most communicable
in the first few days of illness, however infective period continues
since clinical infection may be followed by intermittent and prolonged
rectal shedding
Secondary attack rate within
families is up to 50%;
Adenovrius outbreaks are seasonal: Respiratory disease mainly occurs
in late winter through early summer. Pharyngoconjunctival and EKC
infections occur in the summer months while GI disease does not seem to be
seasonal
Diagnosis
Clinical specimens, such as swabs (nasopharyngeal,
conjuncticval, rectal, or other) and washings, corneal scrapings, stool,
urine or biopsy and autopsy materials etc. should be transported in viral
transport medium.
Viral Isolation in cell cultures is
carried out in
HeLa, human embryonic kidney (HEK) and human fetal diploid cells (HDFL).
A549 cells lines are used for types 1-39.
Subgroup F (serotypes 40, 41) do not
grow well in these cell lines, but do grow in Graham-293 (a modified HEK cell
line).
Shell vial culture technique aids
in faster detection.
Cytopathic effects include
swelling and rounding of cells. Cells may become refractile and clustered into irregular
clumps.
Isolation of virus from a
pharyngeal specimen is more suggestive of a current clinical infection
than from fecal specimen.
Rapid detection of enteric types (serotypes
40, 41) is by ELISA or immunofluorescnece antibody. Immune EM
(aggregation with sera) may also be used
Other detection methods in current
use include electron microscopy, polymerase chain reaction and
nucleic acid probes.
Serology is mainly used for
epidemiologic studies
Prevention
- Hand washing
- Contact precautions, respiratory
precautions in health care settings
- Adequate chlorination of swimming
pools
- Sterilization / disinfection of
ophthalmologic equipment and use of single dose vials of ophthalmic
medications
Vaccine
There is a live, enteric coated,
oral vaccine (against types 4 and 7) which will prevent most
illness caused by these two adenovirus types. The vaccine is only
approved for military personnel 17 through 50 years of age but has side
effects, some of which may be severe. Serious problems have been
reported by about one per cent of vaccinees within six months of
vaccination and include blood in the urine or stool, pneumonia,
inflammation of the stomach or intestines
.
|
|
|
 Return to the Virology section of Microbiology and Immunology On-line
Return to the Virology section of Microbiology and Immunology On-line
This page last changed on
Monday, June 06, 2016
Page maintained by
Richard Hunt
|

 Figure 1. Structure of a paramyxovirus
Figure 1. Structure of a paramyxovirus
 Figure 3.
Figure 3.
 Figure .
Figure .

 Structure of adenovirus
Structure of adenovirus
 Adenovirus
Adenovirus








