|
x |
x |
|
 |
 |
|
INFECTIOUS
DISEASE |
BACTERIOLOGY |
IMMUNOLOGY |
MYCOLOGY |
PARASITOLOGY |
VIROLOGY |
|
TURKISH |
VIROLOGY - CHAPTER SEVENTEEN
VIRAL
AGENTS OF
GASTROENTERITIS
ROTAVIRUSES, CALICIVIRUSES,
ADENOVIRUSES, ASTROVIRUSES AND OTHERS
Dr N. Narayan and Dr Helmut Albrecht
University of South Carolina School of Medicine
Columbia, South Carolina
|
|
Español |
|
|
Let us know what you think
FEEDBACK |
|
SEARCH |
|
|

 |
|
Logo image © Jeffrey
Nelson, Rush University, Chicago, Illinois and
The MicrobeLibrary |
|
|
|
Appendix
Acute Flaccid Myelitis (AFM): Update on Disease Symptoms and Potential
Etiologic Agent(s)
 Figure 1. Rotavirus (A double-capsid particle (left), and a single, inner, capsid (right))
©
Dr Linda
Stannard,
University of Cape Town, South Africa
Figure 1. Rotavirus (A double-capsid particle (left), and a single, inner, capsid (right))
©
Dr Linda
Stannard,
University of Cape Town, South Africa
|
A Large number of
viruses are found in the human gut; these include some that are associated
with gastroenteritis
- Rotaviruses
- Adenoviruses 40/41
- Caliciviruses
- Norwalk-like viruses or small round structured viruses (SRSV)
- Astroviruses
- Small round viruses (SRV)
- Coronaviruses
- Toroviruses
Other viruses found in the gut of a normal individual are not normally
associated with gastroenteritis
- Poliovirus
- Coxsackie A virus
- Coxsackie B Virus
- Echoviruses
- Enteroviruses 68-71
- Hepatitis A virus
- Hepatitis E virus
- Adenoviruses 1-39
- Reoviruses
Others are found in the gut as opportunistic infections
- Cytomegalovirus (CMV)
- Herpes simplex virus (HSV)
- Human immunodeficiency virus (HIV)
ROTAVIRUSES
Classification
The family Reoviridae includes the
genus Rotavirus and Coltivirus (includes Colorado Tick Fever virus). Other
genera include the Orthreoviruses and Orbiviruses (found in sheep).
Rotavirus was first identified by electron
microscopy in 1973 from duodenal biopsies of children with diarrhea. Human and animal rotaviruses are known.
Groups
There are seven different groups (A to G) based on
the antigenicity (each group shares common antigens) and the electrophoretic
mobility of their RNA segments. Groups D, E and F have not been found in
humans. Group A is the most common and only group A rotaviruses cause human disease in the United
States, primarily in the young (under two years of age - infantile
gastroenteritis). However, group A rotaviruses can also cause milder diarrhea in older children and adults. Group B has been found to cause human disease in
China where there may be annual outbreaks of severe adult and infant diarrhea.
More characteristically, group B rotaviruses cause diarrhea in pigs. Group
C is found worldwide.
Serotypes
There are at least 15 different serotypes
of rotaviruses. Fourteen G serotypes are based on G protein (GP7)
differences. Five predominant strains in the United States (G1, G2, G3, G4, G9)
account for 90% of isolates and strain G1 accounts for 73% of infections.
There are 20 P serotypes based on the P protein (VP4) with P4 and P8
predominating.
Common P/G combinations are P8G1, P8G2, P4G2 and P8G4
Structure
Rotaviruses are non-enveloped
viruses with icosahedral symmetry and a double capsid (figure 1). Their electron microscopic appearance shows a 60-80nm
wheel with radiating spokes (Latin, rota = wheel) (figure 2).
The rotavirus genome contains double stranded (ds) RNA
in 11 segments that can be separated by polyacrylamide gel electrophoresis (PAGE).
Major structural proteins
Outer structural proteins are VP7 and VP4. VP4 is the viral hemagglutinin and forms
spikes from the surface.
Inner core structural proteins are VP 1, 2,
3, and 6. VP6 is an important antigenic determinant.
Properties
Rotaviruses are stable in the environment for many months and are
relative resistant to hand washing. They are susceptible to agents such as
95% ethanol, formalin and "Lysol". They are also unstable to pH below
2.
|
|
|

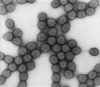 Figure 2. Transmission electron micrograph of intact rotavirus particles,
double-shelled. Distinctive rim of radiating
capsomeres. CDC/Dr. Erskine Palmer
Figure 2. Transmission electron micrograph of intact rotavirus particles,
double-shelled. Distinctive rim of radiating
capsomeres. CDC/Dr. Erskine Palmer
|
Pathogenesis
Affected host cells are mature enterocytes
lining the middle and upper end of the intestinal villi. In laboratory animals,
hepatocytes are also infected. The infectious particle is thought to be
an "intermediate sub-viral particle" (ISVP).
The viral attachment protein is probably
exposed after protease digestion in the GI tract removes some or all of the
outer capsid protein (VP4). Rotaviruses replicate in the host cell
cytoplasm. Virions enter the host cell by endocytosis and viral mRNA is transcribed
using the viral RNA polymerase that is already present in the virion to form
structural protein units of the capsid. The mRNA segments are assembled into
the immature capsid and then replicated to form the double stranded RNA genome.
Large amounts of viral particles are shed in diarrheal stools.
Histopathology of infected intestines
shows villous atrophy and blunting, due to death of the mature enterocytes and
infiltration of lamina propria with mononuclear cells. Subsequently there is
repopulation of the villous tips with immature secretory cells (crypt
hyperplasia).
Cell dysfunction and death results in a net
secretion of intestinal fluid, hence the watery diarrhea. Activation of the
enteric nervous system may also play a role.
Repopulation with immature secretory cells
may contribute to the secondary lactose intolerance that is sometimes seen.
|
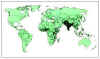 Figure 3A Estimated global distribution of the annual deaths caused by
rotavirus diarrhea. CDC
Figure 3A Estimated global distribution of the annual deaths caused by
rotavirus diarrhea. CDC
 Figure 3B
Figure 3B
National estimates of rotavirus attributable deaths among children under
five years of age (2008)
WHO
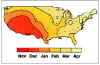 Figure 4. Average time of peak rotavirus activity in the contiguous 48 states,
United States, July 1991 to June 1997 CDC
Figure 4. Average time of peak rotavirus activity in the contiguous 48 states,
United States, July 1991 to June 1997 CDC
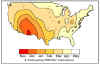 Figure 5. Month of peak rotavirus activity — United States, July 1996–June
1997
Figure 5. Month of peak rotavirus activity — United States, July 1996–June
1997

Figure 6. Average time of peak rotavirus activity in the contiguous 48 states, United States, July 1991 to June
1992. This contour plot was derived using the median value for time of peak activity for each laboratory.
CDC
Higher
resolution movie of above image avi
file
Peak
month for reports of rotavirus infections across the US, 1991-97
avi file
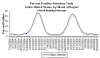 Weekly reports of
rotavirus in the US.
Seasonal variation. CDC
Weekly reports of
rotavirus in the US.
Seasonal variation. CDC
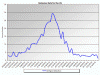 Rotavirus infections weekly trends 2012-2013
Rotavirus infections weekly trends 2012-2013
CDC
|
Epidemiology
Distribution
Rotaviruses are found worldwide,
causing major gastroenteritis and diarrhea-associated hospitalization and
over half a million deaths per year in children under five years of age.
According to WHO, five countries (India, Nigeria, the Democratic Republic of
the Congo, Ethiopia and Pakistan) accounted for more than half of all
rotavirus disease deaths under age five in 2008.
Symptoms include: fever, vomiting, diarrhea and abdominal pain. Seroprevalence studies show that antibody is present in most infants by age
3 years.
Prior to the introduction in the United States of widespread vaccination in
2006, there were up to three million cases of rotavirus infection per year.
In about 1 to 2.5% of cases, there was severe dehydration. This resulted in
20 to 60 deaths of children under five each year. In addition, there were
50,000 to 70,000 hospitalizations and over 500,000 visits to doctors’ offices
per year.
Since the introduction of vaccination there has been a drop in
rotavirus-related hospitalizations by up to 86 percent. It is likely that
vaccination has also protected non-vaccinated infants by limiting
circulating infection. Deaths have also been markedly reduced. In 2008,
there were an estimated 14 deaths from rotavirus disease in the United
States and fewer than 10 in the United Kingdom compared to 98,621 in India.
Seasonality
In the U.S.A., rotavirus infections occur
in the winter months (November through May). The disease spreads across North
America from the warmer climates, starting from Mexico and SW USA and gradually progressing N/NE to reach
East Coast and Canada in spring (figures 4 - 6). As might be expected, rotavirus
infections are seen year round in the tropics.
Incubation period
This is thought
to be less than 4 days
Contagious Period
The patient is contagious from before the onset of diarrhea to a few days
after the end of diarrhea.
Age of infections
Rotaviruses infect children at a young age. Older infants and young children
(4 months - 2 years)
tend to be more symptomatic with diarrhea. Young infants may be protected due to
trans-placental transfer of antibody. Asymptomatic infections are common,
especially in adults. Many cases and outbreaks are
nosocomial
Group A infections are most common.
Group B has been associated with outbreaks
in adults in China
Group C is responsible for sporadic cases
of diarrhea in infants around the world.
Spread
is mainly person to person via fecal - oral route and through
fomites. Spread by
food and water is also possible. There has been speculation that rotaviruses may
also spread via the respiratory route.
High numbers of viral particles are shed
in diarrheal stools (1010/gm). Infective dose is only 10-100 pfu.
|
| |
Clinical Features
Fever can be high grade (>102° F in
30% of patients) and vomiting and nausea precedes diarrhea. Diarrhea is usually watery (no blood or
leukocytes), lasting 3-9 days, but longer in malnourished and immune deficient
individuals. Necrotizing
enterocolitis and hemorrhagic
gastroenteritis is seen in neonates.
Dehydration is the main contributor to
mortality. Secondary malabsorption of lactose and
fat, and chronic diarrhea are possible.
Diagnosis
Rapid diagnosis can be obtained by antigen detection in
stool using ELISA (which uses a monoclonal antibody) and LA. Several kits are
commercially available. These detect only Group A rotavirus. Electron microscopy also detects non-Group A viruses.
Group A rotaviruses can be cultured in
monkey kidney cells.
Epidemiologic studies use patterns of
viral RNA migration by gel electrophoresis (electropherotyping). Different
genetic strains may circulate in a given community.
Treatment
Treatment is just supportive care with rehydration (oral /
intravenous). Antiviral agents not known to be effective.
Prevention of spread
Good hand washing technique is important.
In addition, surfaces, toilets and toys should be disinfected. Adequate chlorination of water
can prevent spread in the community.
Immunity
Antibodies against VP7 and VP4 are
partially protective but the initial infection does not lead to permanent
immunity and reinfection can occur at any age. However, subsequent infections
are usually less severe than the primary infection.
Vaccine
Reassortant vaccines are created by genetic reassortment in which non-human
rotavirus strains express the antigens of human rotaviruses on their surface.
The non-human strains replicate but do not cause disease and are of low
pathogenicity in humans.
A live, tetravalent rhesus-human reassortant
vaccine (Rotashield - Wyeth Laboratories) was first licensed for use in infants in August
1998. It contained human G types 1, 2, 4, and simian G type 3. However, post-licensure surveillance indicated a
possible relationship between the occurrence of
intussusception
3 to 20 days after
the vaccine was administered, especially the first dose (15 cases/1.5 million doses were
reported). Use of the vaccine was suspended and it
was eventually removed from the market in October 1999, when studies confirmed
the link between vaccination and intussusception.
RotaTeq (Merck) is a live oral vaccine licensed in the United States in 2006.
It contains five reassortants (WC3 bovine rotavirus strain with surface proteins
of the G1-4 and P1A human serotypes. It does not contain preservatives or
thimerosal. Three doses are given at 2, 4 and 6 months of age with the minimum
age for the first dose of 6 weeks. The series should not be initiated after 12
weeks. The efficacy of the RotaTeq vaccine is high with 98% reduction in severe rotavirus gastroenteritis within the first year of vaccination and a
96% reduction in hospitalization rate. There is also a 74 and 71% reduction of
rotavirus gastroenteritis within the first and second years after vaccination.
Rotarix (Avant Immunotherapeutics/Glaxo) is a live, attenuated, monovalent
vaccine that contains the G1P[8] human rotavirus strain. It was licensed in the
United States in 2008. It has been studied in South America and has a two dose
schedule of administration. There is no increase in intussuseption. After two
doses, there is protection through the first two years of life.
Hospitalizations are reduced by 96% and severe rotavirus gastroenteritis by
90%. The vaccine is also effective against rotavirus gastroenteritis of any
severity (79%). Significant protection was demonstrated against severe
rotavirus gastroenteritis during two rotavirus seasons caused by types G1 (96%),
G2 (86%), G3 (94%), G4 (95%), and G9 (85%). These are the most commonly
circulating rotavirus types in the United States.
|
|
|
| |
SMALL ROUND RNA VIRAL AGENTS CAUSING
GASTROENTERITIS
This group of RNA viruses morphologically
is subdivided in to 2 sub-groups:
- Structured - Small round structured viruses
(SRSV), Calicivirus, Astrovirus
- Other small viruses that are relatively
structureless or featureless - W (Wollan) and Ditchling.
|
| 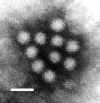 Figure 7. Norwalk virus from stool sample from an individual with gastroenteritis.
Figure 7. Norwalk virus from stool sample from an individual with gastroenteritis.
F.P. Williams, U.S. Environmental Protection
Agency
 Figure 8. Typical morphology of Norwalk-like viruses seen by transmission electron microscopy. The individual virions have a diameter of only 27nm.
Wadsworth Center
of the New York State Department of Health.
Figure 8. Typical morphology of Norwalk-like viruses seen by transmission electron microscopy. The individual virions have a diameter of only 27nm.
Wadsworth Center
of the New York State Department of Health.
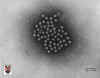 Figure 9. Bovine calcivirus © 1994
Veterinary Sciences Division
- Dr Stewart McNulty at Veterinary Sciences, Queen's University, Belfast.
Figure 9. Bovine calcivirus © 1994
Veterinary Sciences Division
- Dr Stewart McNulty at Veterinary Sciences, Queen's University, Belfast. |
CALICIVIRUSES
Human caliciviruses were first described in 1976.They
belong to the family caliciviridae and are non-enveloped, single stand,
positive sense RNA viruses. They are 27 to 35 nm in size (figure 9). They appear round in shape with icosahedral
symmetry and contain a single capsid protein. The viral surface has 32 cup-shaped depressions (‘calici’=
chalice or calyx i.e. cup-like) described as the ‘Star of David’ appearance.
Otherwise they are similar to Norwalk group of
agents.
Classification
Caliciviruses can be divided into:
- Norwalk and "Norwalk-like" viruses (NLV)
- "Sapporo-like" viruses (SLV)
- Vesiviruses
- Lagoviruses
NLV (Noroviruses) include:
- Norwalk virus
- Hawaii virus
- Snow Mountain virus
- Montgomery County virus
- Taunton (England) virus
SLV (Sapoviruses) include:
- Sapporo virus
- Manchester virus
- Houston/86
- London/92
New types are named after the
place where they were first isolated in relation to outbreaks of diarrhea.
NORWALK VIRUS AND
NORWALK-LIKE VIRAL AGENTS
Norwalk virus
was first detected in stools of patients with gastroenteritis (winter vomiting
disease) in Norwalk, Ohio in 1968. They cause 40 per cent of non-bacterial
gastroenteritis epidemics. Forty five per cent are food-borne and 52 per cent
are raw shell-fish associated. They tend to cause rapid (explosive) epidemics in
places of close contact such as cruise ships, nursing homes, hospitals and
camps. In the electron microscope, these viruses are 27 - 32nm in size with a ragged surface.
Epidemiology
Noroviruses are found world-wide and cause more than 23 million cases of
gastroenteritis very year in the United States. They are the cause of more than
half of gastroenteritis cases in the US. From seroprevalence studies, it has
been found that most people have been infected by the age of four.
There are asymptomatic infections in which the patient is infectious, has
seroconverted and sheds virus. The infective dose may be very low (~10pfu) and
virus may continue to be secreted during the convalescent period. Protective
immunity is short-lived.
Clinical Features
Adults and children are affected.
The infection has a relatively short incubation period of about 24
hours with a range of 12 to 96 hours. The resulting illness is short (less than 3 days).
The most prominent symptoms are is vomiting, nausea, abdominal cramping and
watery diarrhea accompanied by headache, fever and malaise. The 1 to 3 day
period of
diarrhea is less than that associated with rotavirus infections.
Treatment
The symptoms are treated by rehydration and the use of anti-diarrheals.
Complications are rare but can be found in the immunocompromized.
Spread
Norwalk
virus is spread via the feco-oral route and, perhaps, also
through vomit. Outbreaks spread through fecally-contaminated food or water.
Norwalk viruses can survive for several days on plastic surfaces such as counter
tops and telephones and in water that is chlorinated at the usual levels (up to
10 ppm). They can survive freezing and heating to 60 degrees C. They also
survive in steamed shellfish.
Diagnosis
Stool specimens, vomit, suspected food and environmental swabs (during an
outbreak) may be tested using PCR (in state laboratories).
Immune electron microscopy is less
used. Serology may be used for epidemiologic purposes.
Control
CDC recommends disinfection of surfaces using bleach (1 part bleach to 50
parts water). Hand sterilization is also important during an outbreak.
|
|
|
 Figure 10. Astrovirus
© 1994
Veterinary Sciences Division
- Dr Stewart McNulty at Veterinary Sciences, Queen's University, Belfast.
Figure 10. Astrovirus
© 1994
Veterinary Sciences Division
- Dr Stewart McNulty at Veterinary Sciences, Queen's University, Belfast.
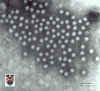 Figure 11. Astrovirus
© 1994 Veterinary Sciences Division
- Dr Stewart McNulty at Veterinary Sciences, Queen's University, Belfast.
Figure 11. Astrovirus
© 1994 Veterinary Sciences Division
- Dr Stewart McNulty at Veterinary Sciences, Queen's University, Belfast.
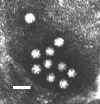 Figure 12. Human astrovirus US
Environmental Protection Agency
Figure 12. Human astrovirus US
Environmental Protection Agency |
ASTROVIRUSES
Astroviruses were described in relation to an outbreak of
gastroenteritis in 1975. They are small single stranded RNA, non-enveloped virus,
about 27 - 32nm
in size. They are round with an unbroken surface (unlike indented surface of calicivirus) (figure 10 - 12).
Their appearance in the electron microscope is a 5 or 6 pointed star within a
smooth edge. They contain 3 structural proteins and their genome has been
sequenced.
Astroviruses are immunologically distinct from Norwalk and
other Caliciviruses - they belong to the family Astroviridae
Eight human serotypes are known and there
are also animal strains.
Clinical Features
Infants, children, immunocompromized patients and the elderly are most often
affected by astrovrius infections. The incubation period is short (1 to 4 days)
and is followed by watery diarrhea, abdominal
cramps, headache, nausea, low-grade
fever, vomiting (the latter being Iess common).
Epidemiology
Astroviruses are endemic worldwide, mainly in children
less than 7 years
of age. Presently, the true disease burden is unclear. Transmission is person-to-person via
fecal-oral route and outbreaks due to fecal contamination of
sea-food or water often occur.
Diagnosis
Electron microscopy and immuno-electron
microscopy are especially useful since
the virus is often shed in large amounts in stool. Immunofluoresence microscopy detects all
serotypes. ELIZA and PCR are also used.
|
|
|
| |
ADENOVIRUSES
Adenoviruses were first isolated in 1953 from adenoidal tissue. The double
stranded DNA viruses about 70 to 75nm in diameter. Mammalian adenoviruses
belong to the genus mastadenovirus. There are six sub-genera of human
adenoviruses (A to F) with 51 serotypes some of which have known oncogenic
potential. In the laboratory, adenoviruses have found use in gene therapy and
vaccine delivery.
Adenovirus serotypes implicated in
gastroenteritis are 40 and 41 which belong to serogroup F. They cause diarrheal disease in infants and
children less than 4 years of age. These ubiquitous viruses are found in the
population year-round and are spread by the
feco-oral
route. They are not shed in the nasopharynx.
The incubation period of adenoviral
gastroenteritis is 3 to 10 days and diarrhea lasts 10 to 14 days; prolonged
diarrhea often seen with type 40 infections. This can also lead to
intussuseption, mesenteric adenitis and appendicitis.
Isolation requires a special medium, Graham 29.
Diagnosis is made by latex agglutination
and ELISA tests or by electron microscopy.
|
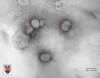 Figure 13
Figure 13
Torvirus negative stain electron microscopy. © Stewart
McNulty, Queens University, Belfast. |
TOROVIRUSESToroviruses (figure 13) belong to the family
Coronaviridae and the genus
Torovirus
They are pathogens for both humans and animals. They are pleiomorphic, coated,
single positive strand RNA viruses. In the electron microscope they have a
doughnut shape (torus). They cause watery diarrhea in infants of 2 to 12 months.
They are usually diagnosed by electron microscopy.
|
| |
CYTOMEGALOVIRUS
These are herpes viruses
which in normal people give rise to a number of diseases, particularly
infectious mononucleosis in western countries. In the immunocompromized, they
lead to retinitis, hepatitis and colitis.
|
|
|
 Return to the Virology section of Microbiology and Immunology On-line. Return to the Virology section of Microbiology and Immunology On-line.
 Return to the Home Page of Microbiology and Immunology On-line
Return to the Home Page of Microbiology and Immunology On-line
This page last changed on
Friday, December 28, 2018
Page maintained by
Richard Hunt
|


 Figure 3A Estimated global distribution of the annual deaths caused by
rotavirus diarrhea. CDC
Figure 3A Estimated global distribution of the annual deaths caused by
rotavirus diarrhea. CDC
 Figure 10. Astrovirus
© 1994
Veterinary Sciences Division
- Dr Stewart McNulty at Veterinary Sciences, Queen's University, Belfast.
Figure 10. Astrovirus
© 1994
Veterinary Sciences Division
- Dr Stewart McNulty at Veterinary Sciences, Queen's University, Belfast.
 Figure 13
Figure 13










