|
TEACHING
OBJECTIVES
Know the
distinction between passive and active immunization and their examples
Distinguish
between artificial and natural means of immunization
Know the
applications and problems of artificial passive immunization
Know the
applications and problems of artificial active immunization
Know the
modern approaches to immunization |
Immunization is a means of providing specific protection against many common
and damaging pathogens by stimulating an organism's immune system to either
produce humoral antibodies against the pathogen (or toxins produced by the
pathogen) or T cells that can provide cell-mediated immunity.
The type of immunity that is needed to neutralize a specific pathogen
depends on the site of the pathogen and the mechanism of its pathogenesis.
For example, some pathogens produce disease by secreting
exotoxins.
If this is the case, the only immune mechanism effective against the
organism would be neutralizing antibodies that prevent exotoxin binding to
the appropriate receptor on its target cell and promoting its clearance and
degradation by phagocytes.
If the pathogen produces disease by other means, an antibody will have to
react with the pathogen itself and eliminate it either by
complement-mediated lysis
or phagocytosis and intracellular killing. However, if the pathogenic
organism is localized intracellularly, it will not be accessible to
antibodies and the cell harboring it will have to be destroyed instead; only
then could antibody have any effect on the pathogen. Most viruses,
together with intracellular bacteria and protozoa, are examples of such
pathogens. In this case, the harboring cells can be destroyed by elements of
cell-mediated immunity or, if they cause the infected cell to
express unique antigens recognizable by antibody, antibody-dependent and
complement-mediated killing of the infected cell can expose the pathogen to
elements of humoral immunity. It is also possible for cells harboring
intracellular pathogen to be activated to kill the pathogen. Such is clearly
not the case with pathogens that have the capability of surviving within
phagocytic cells.
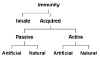
Specific immunity can result from either passive or active immunization and both
modes of immunization can occur by natural or artificial processes (Figure 1C).
Passive Immunity
Immunity can be acquired, without the
immune system being challenged with an antigen. This is done by transfer of serum or
gamma-globulins from an immune donor to a non-immune individual. Alternatively,
immune cells from an immunized individual may be used to transfer immunity.
Passive immunity may be acquired naturally or artificially.
Naturally acquired passive immunity
Immunity is transferred from mother to fetus through placental transfer of IgG
or colostral transfer of
IgA.
Artificially acquired passive immunity
Immunity is often artificially transferred by injection with gamma-globulins
from other individuals or gamma-globulin from an immune animal. Passive transfer
of immunity with immune globulins or gamma-globulins is used in numerous
acute situations of infection (diphtheria, tetanus, measles, rabies, etc.),
poisoning (insects, reptiles, botulism), and as a prophylactic measure (hypogammaglobulinemia).
In these situations, gamma-globulins of human origin are preferable, although
specific antibodies raised in other species are effective and used in some cases
(poisoning, diphtheria, tetanus, gas gangrene, botulism). While this form of
immunization has the advantage of providing immediate protection, heterologous
gamma-globulins are effective for only a short duration and often result in
pathological complications (serum sickness) and
anaphylaxis. Homologous immunoglobulins also carry the risk of transmitting hepatitis and HIV.
Passive transfer of cell-mediated
immunity can also be accomplished in certain diseases (cancer,
immunodeficiency). However, it is difficult to find histocompatible
(matched) donors and there is severe risk of graft versus host disease.
|

Figure 1A. Edward Jenner carries out a vaccination
 B. Pre and post vaccine incidence of common infectious diseases
B. Pre and post vaccine incidence of common infectious diseases
 C. Modes of immunization
C. Modes of immunization
 D. Milestones of immunization
D. Milestones of immunization

Figure 2 Introduction of variolation
 Figure 3
Figure 3
Live attenuated vaccines
 Figure 4 Killed whole organism vaccines
Figure 4 Killed whole organism vaccines

 Figure 5
Figure 5
Microbial fragment vaccines
 Figure 6 Modification of toxin to toxoid
Figure 6 Modification of toxin to toxoid

Figure 7 Advantages and disadvantages of passive immunization
|
Active Immunity
This refers to immunity produced by
the body following exposure to antigens.
Naturally acquired active immunity
Exposure to various pathogens leads to sub-clinical or clinical infections
which result in a protective immune response against these pathogens.
Artificially acquired active immunity
Immunization may be achieved by administering live or dead pathogens or their
components. Vaccines used for active immunization consist of live (attenuated)
organisms, killed whole organisms, microbial components or secreted toxins
(which have been detoxified).
Live vaccines
The first live vaccine was
cowpox virus introduced by Edward Jenner as a vaccine for smallpox (see
vaccine
section); however,
variolation
(innoculation using pus from a
patient with a mild case of smallpox) has been in use for over a thousand
years (figure 2)
Live vaccines are used against a number of viral infections
(polio (Sabin vaccine), measles, mumps, rubella, chicken pox, hepatitis A, yellow fever,
etc.)
(figure 3).
The only example of live bacterial vaccine is one against tuberculosis (Mycobacterium
bovis: Bacille Calmette-Guerin vaccine: BCG). This is is used in many
African, European and Asian countries but not in the United States. Whereas many studies have
shown the efficacy of BCG vaccine, a number of studies also cast doubt on its
benefits.
Live vaccines normally produce self-limiting
non-clinical infections and lead to subsequent immunity, both humoral
and cell-mediated, the latter being essential for intracellular
pathogens. However, they carry a serious risk of causing overt disease
in immunocompromised individuals. Furthermore, since live vaccines are
often attenuated (made less pathogenic) by passage in animals or thermal
mutation, they can revert to their pathogenic form and cause serious
illness. It is for this reason that live polio (Sabin) vaccine, which was
used for many years, has been replaced in many countries by the
inactivated (Salk) vaccine.
Killed vaccines
Killed (heat, chemical
or UV irradiation) viral vaccines include those for polio (Salk vaccine), influenza,
rabies, influenza, rabies, etc. Most bacterial vaccines are killed organisms (typhoid, cholera, plague, pertussis,
etc.) (figure 4).
Sub-unit vaccines
Some anti-bacterial vaccines utilize purified cell wall components (haemophilus, pertussis, meningococcus,
pneumococcus, etc.) (figure 5). Some viral vaccines (hepatitis-B, etc.)
consist of purified antigenic proteins manufactured after expression from a gene cloned into a suitable vector (e.g.,
yeast). When the pathogenic mechanism of an agent involves a toxin, a modified
form of the toxin (toxoid, which has lost its toxicity while remaining
immunogenic) is used as a vaccine (e.g., diphtheria, tetanus,
cholera) (figure 6). These subunit vaccines are designed to reduce the toxicity problems.
Each type of vaccine has its own advantages and disadvantages (figure 7).
Subunit vaccines may consist of proteins or polysaccharides. Since polysaccharides
are relatively weak T-independent antigens, and produce only IgM
responses without immunologic memory, they are made more immunogenic and
T-dependent by conjugation with proteins (e.g., haemophilus,
meningococcus, pneumococcus, etc.).
Other novel vaccines
A number of novel approaches to active immunization are in the
investigative stage and are used only experimentally. These include
anti-idiotype antibodies, DNA vaccines and immunodominant peptides
(recognized by the MHC molecules) and may be available in the future.
-
Anti-idiotype antibodies against polysaccharide antibodies produce long
lasting immune responses with immunologic memory.
-
DNA vaccines (viral peptide genes
cloned into vectors) must be injected. They transfect host cells and consequently
produce a response similar to that produced against live-attenuated
viruses (both cell-mediated and humoral). Several anti-HIV DNA vaccines
have been developed but none has so far shown much efficacy.
-
Immunodominant peptides are
simple and easy to prepare and, when incorporated into MHC polymers, can
provoke both humoral and cell mediated responses.
Adjuvants
Weaker antigens may be rendered more immunogenic by the addition of
other chemicals. Such chemicals are known as adjuvants. There are many
biological and chemical substances that have been used in experimental
conditions (Table 1). However, only aluminum salts (alum) are approved
for human use and it is incorporated in
DTP vaccine. Furthermore, pertussis itself has adjuvant effects. Adjuvants used experimentally
include mixtures of oil and detergents, with (Freundís complete
adjuvant) or without (Freundís incomplete adjuvant) certain bacteria.
Bacteria most often used in an adjuvant are Mycobacteria (BCG) and
Nocardia. In some instances, sub-cellular fractions of these bacteria can
also be used effectively as adjuvants. Newer adjuvant formulations
include synthetic polymers and oligonucleotides. Most adjuvants
recognize TOLL-like receptors, thus activating mononuclear phagocytes and
inducing selective cytokines that can enhance Th1 or Th2 responses,
depending on the nature of the adjuvant.
|
Table 1.
Selected adjuvants in clinical or experimental use |
|
Adjuvant type |
Human use |
Experimental only |
|
Salts:
aluminum hydroxide, aluminum phosphate-calcium phosphate
|
Yes
Yes |
Slow
release of antigen, TLR interaction and cytokine induction |
|
Beryllium hydroxide
|
No
|
|
Synthetic particles:
Liposomes, ISCOMs,
polylactates
|
No
No
|
Slow
release of antigen |
|
Polynucleotides:
CpG and others
|
No* |
TLR
interaction and cytokine induction |
|
Bacterial products:
B.pertussis
|
Yes |
TLR
interaction and cytokine induction |
M. bovis
(BCG and others)
|
No |
Mineral oils
|
No |
Antigen depot |
|
Cytokines:
IL-1, IL-2, IL12,
IFN-γ, etc. |
No* |
Activation and
differentiation of T- and B cells and APC |
*Experimental use
in human malignancies
|
The protective immunity conferred by a vaccine may be
life-long (measles, mumps, rubella, small pox, tuberculosis, yellow
fever, etc.) or may last as little as a few months (cholera). The
primary immunization may be given at the age of 2 to 3 months (diphtheria, pertussis, tetanus,
polio), or 13
to 15
months (mumps, measles, rubella). The currently recommended schedule
for
routine immunization in the United States (recommended by CDC and AIP) is
summarized in Table 2. This schedule is revised on a yearly basis or as
need by the CDC Advisory Committee on Immunization Practice (AICP).
|
Table
2 Schedule for Active Immunization of Normal Children* |
|
Age
Vaccine
|
Birth |
Months |
Years |
|
1 |
2 |
4 |
6 |
12 |
15 |
18 |
19 -23 |
2-3 |
4-6 |
|
Hepatitis-B 1 |
HeB |
HeB |
1 |
HeB |
|
|
HeB |
|
Rotavirus 2 |
|
|
Rota |
Rota |
Rota |
|
|
|
|
|
|
Diphtheria, Tetanus,
Pertussis 3 |
|
|
DTaP |
DTaP |
DTaP |
3 |
DTaP |
|
|
DTaP |
|
Hemophilus influenzae-b
(CV) 4 |
|
|
Hib |
Hib |
Hib4 |
Hib |
|
|
Pneumococcal 5 |
|
|
PCV |
PCV |
PCV |
PCV |
|
PPV |
Inactivated
Poliovirus |
|
|
IPV |
IPV |
IPV |
|
|
IPV |
|
Influenza 6 |
|
|
|
|
Influenza (yearly) |
|
|
Measles, Mumps, Rubella
7 |
|
|
MMR |
|
|
MMR |
MMR |
|
Varicella
8 |
|
|
Var |
|
|
|
|
|
Hepatitis A 9 |
|
|
|
|
|
Hep A (2 doses) |
HepA
series |
|
Meningococcal 10 |
|
|
|
|
|
|
MCV4 |
|
*Recommended by Advisory
Committee on Immunization , American academy of Pediatrics
|
Range of recommended ages |
Certain high risk groups |
CDC
Immunization
schedules |
|

Adverse events occurring with 48 hours of DPT vaccination |
Prophylactic versus
therapeutic immunization
Most vaccines are given prophylactically, i.e. prior to exposure to the
pathogen. However, some vaccines can be administered therapeutically, i.e.
post exposure (e.g., rabies virus). The effectiveness of this mode of
immunization depends on the rate of replication of the pathogen, incubation
period and the pathogenic mechanism. For this reason, only a booster shot with
tetanus is sufficient if the exposure to the pathogen is within less than 10
years and if the exposure is minimal (wounds are relatively superficial). In a
situation where the pathogen has a short incubation period, only a small amount
of pathogenic molecules could be fatal (e.g., tetanus and diphtheria); therefore both
passive and active post exposure immunization are essential. This is also the
case when a bolus of infection is relatively large
Passive
prophylactic immunization is also normal in cases of defects in the immune
system, such as hypogammaglobulinemias.
Adverse effects of
immunization
Active immunization may cause fever, malaise and discomfort. Some vaccine may
also cause joint pains or arthritis (rubella), convulsions, that may sometimes
be fatal (pertussis),
or neurological disorders (influenza). Allergies to eggs may develop as a
consequence of viral vaccines produced in eggs (measles, mumps, influenza, yellow
fever). Booster shots result in more pronounced inflammatory effects than the
primary immunization. The serious side effects have been documented after use of
the DTP vaccine (Table 3). Most of these were attributable
to the whole pertussis component of the vaccine and have been eliminated by the
use of an acellular pertussis preparation.
|
Table 3.
Approximate rates of adverse event occurring within 48 hours DTP
vaccination |
|
Event |
Frequency |
|
Local |
| Redness,
swelling, pain |
1
in 2-3 doses |
|
Mild/moderate systemic |
| Fever,
drowsiness, fretfulness |
1
in 2-3 doses |
| Vomiting,
anorexia |
1
in 5-15 doses |
|
More serious systemic |
| Persistent
crying, fever |
1
in 100-300 doses |
| Collapse,
convulsions |
1
in 1750 doses |
| Acute
encephalopathy |
1
in 100,000 doses |
| Permanent
neurological deficit |
1
in 300,000 doses |
|













