|
c |
c |
|
 |
 |
|
INFECTIOUS
DISEASE |
BACTERIOLOGY |
IMMUNOLOGY |
MYCOLOGY |
PARASITOLOGY |
VIROLOGY |
|
SPANISH |
BACTERIOLOGY - CHAPTER EIGHTEEN
BORDETELLA, HAEMOPHILUS AND
LEGIONELLA
Dr Abdul Ghaffar
Professor Emeritus
University of South Carolina School of Medicine
|
|
SLOVAK |
|
TURKISH |
|
ALBANIAN |
|
Let us know what you think
FEEDBACK |
|
SEARCH |
|
|
|

 |
|
|
|
|
|
Logo image © Jeffrey
Nelson, Rush University, Chicago, Illinois and
The MicrobeLibrary |
|
|
| TEACHING OBJECTIVES
To know the general morphology and
physiology the organisms
To know epidemiology and clinical
symptoms
To understand the mechanisms
pathogenesis
To know the diagnostic,
therapeutic and preventive procedures |
BORDETELLA
Bordetella pertussis is the only organism of major
clinical significance within this genus; it causes whooping cough in infants and young children.
However, a closely related organism, B. parapertussis can also cause a
milder form of bronchitis. B. bronchosepticus, another member of the
genus Bordetella, is the causative agent of respiratory diseases in cats
and swine, but can cause broncho-pulmonary symptoms in severely immunosupressed
individuals.
Bordetella pertussis
Morphology and physiology
B. pertussis is an extremely small,
strictly aerobic, Gram negative, non-motile cocobacillus (short rod). Compared
to other Bortdetella species, B. pertussis does not grow on common
laboratory media and can be distinguished from B. parapertussis in that
B.
pertussis is oxidase positive but urease negative, while B. parapertussis
is oxidase negative and urease positive. B. bronchosepticus is positive
for both enzymes.
|
|
|
 Photomicrograph of Bordetella (Haemophilus) pertussis bacteria using
Gram stain technique. CDC
Photomicrograph of Bordetella (Haemophilus) pertussis bacteria using
Gram stain technique. CDC
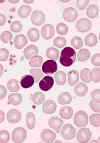 BLOOD LYMPHOCYTOSIS IN A PATIENT WITH PERTUSSIS. The lymphocytes in this blood smear from an 18-month-old
child with a Bordetella pertussis infection have lobulated nuclei. Lymphocytosis is characteristic of this disorder and the lymphocyte
morphology is often atypical. The cytology of the cells could be mistaken for neoplastic lymphocytes.
(Wright-Giemsa stain) © The Johns Hopkins Autopsy Resource
(JHAR). Image Archive.
BLOOD LYMPHOCYTOSIS IN A PATIENT WITH PERTUSSIS. The lymphocytes in this blood smear from an 18-month-old
child with a Bordetella pertussis infection have lobulated nuclei. Lymphocytosis is characteristic of this disorder and the lymphocyte
morphology is often atypical. The cytology of the cells could be mistaken for neoplastic lymphocytes.
(Wright-Giemsa stain) © The Johns Hopkins Autopsy Resource
(JHAR). Image Archive.
 Pertussis in the US, 1940-1999 CDC
Pertussis in the US, 1940-1999 CDC
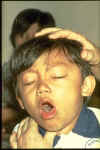 This child has pertussis. It is difficult for him to stop coughing and to get air.
Coughing spasms with a "whooping" sound that follows the cough are typical.
The sound means child is trying to catch his breath before the next round of coughing
© WHO
This child has pertussis. It is difficult for him to stop coughing and to get air.
Coughing spasms with a "whooping" sound that follows the cough are typical.
The sound means child is trying to catch his breath before the next round of coughing
© WHO |
Epidemiology and symptoms
Most of the patients with
whooping cough are less than a year old although older children may also get the
disease. The severity of disease is also age-related. The organism, contained in
aerosol droplets, gains access via inhalation and colonizes the bronchial
ciliary epithelial cells. After a week to 10 days of incubation period, mild
symptoms of rhinitis, mild cough and sneezing occur (catarrhal stage)
which last 1-2 weeks. Further proliferation of the organism compromise
ciliary function and is accompanied by increased frequency and intensity of
symptoms. This leads to the paroxysmal stage, characterized by paroxysms
of cough followed by a prolonged and distressing inspiratory gasp (whoop). The
cough, which recurs at variable intervals and often every few minutes, may last
for 2-3 weeks. The cough interferes with oral intake, and the swallowed mucus
may induce vomiting, resulting in severe dehydration and weight loss. Hypoxia
during prolonged attacks may lead to seizure, hypoxic encephalopathy or coma.
The cough episodes slowly decrease and there is gradual recovery over 3-16 weeks
(convalescent stage). Pneumonia (due to B. pertussis or other
bacterial pathogens), otitis media, rectal prolapse and meningo-encephalitis are
among the secondary complications.
Pathogenesis
The symptoms following the infection are
due to many factors. In addition to the attachment to and growth on ciliated
cells, the organism produces a number of
exotoxins which contribute to these
symptoms.
Pertussis toxin
(pertussigen)
Pertussis toxin is an oligopeptide
AB-type exotoxin that is the major cause of pertussis (abnormal cough). It
causes T cell
lymphocytosis and has
adjuvant properties. It also causes
hypoglycemia, increased IgE synthesis, and increased histamine and
endotoxin
sensitivity. The organism inhibits many leukocyte functions, including
chemotaxis, phagocytosis and respiratory burst and impairs NK cell killing. It
also contributes to bacterial binding to ciliated epithelial cells. It exerts
many of its effects by covalent addition of ADP-ribose to the GTP binding Gi
protein and thereby preventing the deactivation of adenylate cyclase. This
results in the accumulation of large amounts of cAMP which leads to increased
mucus secretion and interferes with many cellular functions.
Adenylate cyclase toxin
This exotoxin penetrates the
host cells, is activated by
calmodulin and catalyzes the conversion of ATP to
cAMP. Like pertussigen, it also inhibits phagocyte and NK cell functions.
However, in contrast with pertussigen, the cAMP increase caused by this toxin is
short-lived.
Tracheal cytotoxin
This is a peptidoglycan-like molecule
(monomer) which binds to ciliated epithelial cells, thus interfering with
ciliary movement. In higher concentrations, it causes ciliated epithelial cell
extrusion and destruction. The destruction of these cells contributes to
pertussis.
Dermonecrotic (heat-labile) toxin
Dermonecrotic toxin is a very strong
vaso-constrictor and causes ischemia and extravasation of leukocytes and, in
association with tracheal cytotoxin, causes necrosis of the tracheal tissue.
Filamentous haemagglutinins (agglutinogens)
These are
not exotoxins but are filament-associated lipo-oligo-saccharides which are
implicated in the binding of the organism to ciliated epithelial cells.
Antibodies against these molecules are protective, probably by preventing
bacterial attachment.
|
 Binding of pertussis toxin to cell membrane
Binding of pertussis toxin to cell membrane |
Lipopolysaccharide (LPS)
Like LPS of other gram negative
bacteria, these endotoxins cause a number of patho-physiolocigal effects. When
released in relatively large quantities following bacterial cell lysis, they
cause irreversible shock and cardiovascular collapse. In smaller quantities,
they activate a variety of inflammatory mediators ( TNF, IL1, IL6,
prostaglandins, etc.) and generate complement activation products.
Diagnosis
Symptoms are characteristic. Laboratory
diagnosis is made by obtaining a nasopharyngeal aspirate and primary culture on
Bordet-Gengou medium (potato-glycerol-blood agar). Growth is inhibited by
peptones, unsaturated fatty acids, sulphides, etc. found in ordinary
media. The organism grows as small transparent hemolytic colonies. It can be
serologically distinguished from B. parapertussis and B.
bronchosepticus.
Prevention and treatment
A killed whole bacterial vaccine
is normally administered as DPT combination. An acellular vaccine consisting of
filamentous hemagglutinins and detoxified pertussigen is also available and is
recommended for booster shots. Erythromycin is the current drug of choice.
|
|
VIDEO
Baby with pertussis
Infant with pertussis
Toddler with pertussis
Child with
pertussis
Courtesy of California Department
of Health Services and Healthy Nevadans 2000, Nevada State Health Division
and
Immunization Action Coalition
Real Video |
 Incidence of H. influenzae non-type b invasive disease among children <5 years of age,
1996. CDC/Barbara Rice ber2@cdc.gov
Incidence of H. influenzae non-type b invasive disease among children <5 years of age,
1996. CDC/Barbara Rice ber2@cdc.gov |
HAEMOPHILUS
The genus
Haemophilus contains many species but H.
influenzae is the most common pathogen. Other species of Haemophilus
that are of clinical importance to immuno-competent humans are H. ducreyi
(causes chancroid: an STD), H. influenzae aegyptius (associated with
conjunctivitis and Brazilian purpuric fever) and H. parainfluenzae (a
rare cause of pneumonia and
endocarditis). There are several species of Hemophilus
that are normal flora, but may be pathogenic in immuno-compromised hosts. The
capsulated strain of H. influenzae (type b) is most virulent, although
some non-encapsulated (non typable) strains are also pathogenic.
Haemophilus influenzae
Morphology and physiology
|
|
|
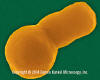 Haemophilus influenzae - coccobacillus prokaryote (dividing); causes meningitis in children, pneumonia,
epiglottitis, laryngitis, conjunctivitis, neonatal infection, otitis media (middle ear infection) and sinusitis in adults
(SEM x 64,000)
©
Dennis Kunkel Microscopy, Inc.
Used with permission
Haemophilus influenzae - coccobacillus prokaryote (dividing); causes meningitis in children, pneumonia,
epiglottitis, laryngitis, conjunctivitis, neonatal infection, otitis media (middle ear infection) and sinusitis in adults
(SEM x 64,000)
©
Dennis Kunkel Microscopy, Inc.
Used with permission |
H. influenzae is a small Gram
negative bacillus which can be grown on chocolate agar (heated blood) and
requires hemin (factor X) and nicotinamide adenine dinucleotide (NAD+:factor
V) for growth which is enhanced by high CO2 concentration (5%). It
does not grow on normal blood agar. The factor V and factor X requirement can be
used to distinguish between H. influenzae which requires both, H.
parainfluenzae which requires factor V only and H. ducreyi which
requires factor X only. H. influenzae are divided into several strains on
the basis of capsular polysaccharides (a-f) or the absence of a capsule (non-typable).
Epidemiology and symptoms
H. influenzae causes a
variety of clinical symptoms some of which may depend on the presence of the
bacterial capsule. Until the availability of the Hib vaccine, the type-b H.
influenzae was the main cause of meningitis in children between 6 months and
5 years, although older children, adolescents and adults can also be infected.
The infection initially causes a runny nose, low grade fever and headache (1-3
days). Due to its invasive nature the organism enters the circulation and
crosses the blood-brain barrier, resulting in a rapidly progressing meningitis
(stiff neck), convulsions, coma and death. Timely treatment may prevent coma and
death, but the patient may still suffer from deafness and mental retardation.
Type-b H. influenzae may also cause septic arthritis conjunctivitis,
cellulitis, and
epiglottitis, the latter results in the obstruction of the upper
airway and suffocation. H. influenzae of other types may rarely cause
some of the symptoms listed above. Non-typable strains of H. influenzae
are the second commonest cause of
otitis media in young children (second to
Streptococcus
pneumoniae). In adults, these organisms cause pneumonia, particularly in
individuals with other underlying pulmonary infections. These organisms also
cause acute or chronic sinusitis in individuals of all ages.
|
 Clinical symptoms of infection by Haemophilus
Clinical symptoms of infection by Haemophilus
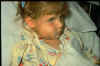 This child has swollen face due to Hib infection.
The tissue under the skin covering the jaw and cheek is infected.
Infection is spreading into her face. She is probably very sick Courtesy of Children's Immunization Project, St. Paul, MN
This child has swollen face due to Hib infection.
The tissue under the skin covering the jaw and cheek is infected.
Infection is spreading into her face. She is probably very sick Courtesy of Children's Immunization Project, St. Paul, MN
 Gross pathology of subacute bacterial endocarditis involving mitral valve.
Left ventricle of heart has been opened to show mitral valve fibrin vegetations due to infection with Haemophilus
parainfluenzae. Autopsy. CDC/Dr. Edwin P. Ewing, Jr. epe1@cdc.gov
Gross pathology of subacute bacterial endocarditis involving mitral valve.
Left ventricle of heart has been opened to show mitral valve fibrin vegetations due to infection with Haemophilus
parainfluenzae. Autopsy. CDC/Dr. Edwin P. Ewing, Jr. epe1@cdc.gov
|
Pathogenesis
The exact mechanism of pathogenesis is not known but the
presence of capsule, which is anti-phagocytic, is a major factor in virulence.
Type-b H. influenzae are more invasive and pathogenic than other strains.
The lipopolysaccharide is responsible for the inflammatory process. The
organisms also produce IgA1-specific protease which may aid their mucosal
colonization.
Diagnosis
Presumptive diagnosis is based on history,
physical examination and symptoms. Blood cultures are positive in more than 50%
of symptomatic patients, except those with conjunctivitis. Polyribitol phosphate
(PRP), a component of the capsular polysaccharide is present in the serum,
cerebrospinal fluid (CSF) and concentrated urine of more than 95% of H.
influenzae-b meningitis cases. Gram-negative cocobacilli can be found in the
CSF in more than 80% of meningitis cases. Some Gram-stained preparations may be
useful in rapid diagnosis of septic arthritis and lower respiratory diseases.
Treatment and prevention
Unless prompt treatment is
initiated, H. influenzae-b meningitis and epiglotitis are almost 100%
fatal. Due to common resistance to ampicillin and some resistance to
chloramphenicol, cephalosporin, which penetrates the blood brain barrier, is the
antibiotic of choice in these cases. Other diseases caused by this organism can
be treated with ampicillin (if susceptible) or choice of
trimethoprim-sulphamethoxazol, tetracyclin and
cefaclor.
Hib-C vaccine which consists of capsular PRP conjugated to
tetanus toxoid has been used successfully to provide protection and is a part of
the recommended routine vaccination schedule.
|
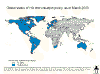 Countries implementing routine childhood Hib immunization © WHO
Countries implementing routine childhood Hib immunization © WHO
 Legionella pneumophila multiplying inside a cultured
cell. Multiple intracellular bacilli, including dividing bacilli, are visible in longitudinal and cross section. Transmission electron micrograph.
CDC/Dr. Edwin P. Ewing, Jr.
Legionella pneumophila multiplying inside a cultured
cell. Multiple intracellular bacilli, including dividing bacilli, are visible in longitudinal and cross section. Transmission electron micrograph.
CDC/Dr. Edwin P. Ewing, Jr.
 Legionella pneumophila. Rod-Shaped Bacterium (SEM x22,810)
©
Dennis Kunkel Microscopy, Inc.
Used with permission
Legionella pneumophila. Rod-Shaped Bacterium (SEM x22,810)
©
Dennis Kunkel Microscopy, Inc.
Used with permission
 Legionella growing on an agar plate with enriched nutrients and charcoal. The iridescent sheen of the colonies as well as the apparent "cut-glass" appearance is characteristic of this species. A confirmed identification would be made by direct fluorescent antibody (DFA) technique.
© Gloria J. Delisle and Lewis Tomalty, Queens University, Kingston, Ontario
Canada and The
MicrobeLibrary
Legionella growing on an agar plate with enriched nutrients and charcoal. The iridescent sheen of the colonies as well as the apparent "cut-glass" appearance is characteristic of this species. A confirmed identification would be made by direct fluorescent antibody (DFA) technique.
© Gloria J. Delisle and Lewis Tomalty, Queens University, Kingston, Ontario
Canada and The
MicrobeLibrary
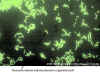 DFA technique to detect the Legionella antigen directly in patient specimens. Respiratory tract specimens are spread on a glass slide. A
monoclonal antibody to Legionella that is tagged with a fluorescein dye is added to the slide. If the antigen is present, the antibody will bind and the outline of the bacilli can be
detected by microscopy under UV light.
©
Gloria J. Delisle and Lewis Tomalty, Queens University, Kingston, Ontario Canada
and The MicrobeLibrary
DFA technique to detect the Legionella antigen directly in patient specimens. Respiratory tract specimens are spread on a glass slide. A
monoclonal antibody to Legionella that is tagged with a fluorescein dye is added to the slide. If the antigen is present, the antibody will bind and the outline of the bacilli can be
detected by microscopy under UV light.
©
Gloria J. Delisle and Lewis Tomalty, Queens University, Kingston, Ontario Canada
and The MicrobeLibrary
|
Haemophilus ducreyi
This is a significant cause of genital ulcers (chancroid) in
Asia and Africa but, is seen less commonly in the United States. The incidence
is approximately 4000-5000 per year with clusters found in California, Florida,
Georgia and New York. The infection is asymptomatic in women but about a week
following sexual transmission to a man, it causes appearance of a tender papule
with erythematous base on the genitalia or the peripheral area. The lesion
progresses to become a painful ulcer with inguinal lymphadenopathy. The H.
ducreyi lesion (chancroid) is distinguished from a syphilitic lesion (chancre) in that it is a comparatively soft lesion. The organism is more
fastidious than H. influenzae but can be grown on chocolate agar,
supplemented with IsovitaleX in 5%-10% CO2 atmosphere and the growth
can be detected in 2-4 days.
Haemophilus influenzae aegyptius
This bacterium, previously known as H. aegyptius,
causes an opportunistic organism which can result in a fulminant pediatric
disease (Brazilian purpuric fever) characterized by an initial
conjunctivitis, followed by an acute onset of fever, accompanied by vomiting and
abdominal pain. Subsequently, the patient develops
petechiae,
purpura, shock and
may face death. The pathogenesis of this infection is poorly understood. The
growth conditions for this organism are the same as those for H. influenzae.
Both H. ducreyi and H. influenzae aegyptius can
be treated with erythromycin.
LEGIONELLA
In 1976, Legionella pneumophila was recognized as a
newly described pathogen after an outbreak of pneumonia among a group of
Legionnaires at a convention in Philadelphia. The disease was subsequently
referred to as Legionnaires' disease. Another flu-like form of the disease
is referred to as Pontiac fever. L. pneumophila is now recognized as
a ubiquitous aquatic saprophyte which causes epidemics and sporadic
infections. The organisms are spread via aerosols and no person to person
transmission has been reported.
Legionellae are facultative intracellular pathogens,
which stain poorly as Gram negative rods. The causative agent was not
recognized previously, since it does not grow on conventional agar such as
sheep blood agar. Nowadays L. pneumophila is cultured on medium that
contains iron and cysteine which are vital for growth (e.g. charcoal yeast
extract agar). However, primary isolation is still difficult from clinical
specimens.
Organisms of Clinical
Importance
After recognition of their unique culture
characteristics, a large number of other species of Legionella
were isolated from environmental and clinical samples. These organisms
are only occasional causes of human disease and the vast bulk of
legionellosis is caused by Legionella pneumophila (most are
serogroup 1 and 6).
The second most common cause of pneumonia is
Legionella micdadei. This organism also stains weakly acid fast on
primary isolation, but loses this property in vitro. This does
not mean that it is anyway related to the Mycobacteria.
Microbiology
Legionellae are poorly staining Gram negative
rods which are identified by growth on buffered charcoal yeast extract (BCYE),
and require L-cysteine and iron for growth. The organisms are fairly
slow growing requiring 3 to 7 days at 35 degrees. Colonies are small
with a ground glass appearance.
The Center for Disease Control (CDC) lists four tests
for the identification of Legionnaires' disease:
PCR tests for L. pneumophila in clinical
specimens are available; however the CDC does not recommend the routine
use of genetic probes or PCR for detection in clinical samples.
Public Health
Legionella pneumophila is an organism that resides in the
environment in pools of stagnant water worldwide. It is found as an
intra-cellular agent within protozoa and a component of biofilms.
Legionnaires' disease is recognized as a sporadic infection, often
associated with travel, an epidemic disease of community-acquired
pneumonia and a nosocomial infection. It often infects hot water towers
and air conditioning systems. When found in buildings, anti-bacterial
treatment of the water supply is recommended. One recently identified
source of Legionella infections is the water used in car
windscreen washers, the reservoirs being warmed by the car engine. The
use of windscreen washer fluid (which contains methanol) solves this
problem.
The organism is transmitted in contaminated air but not
spread person-person. Legionellosis is listed as one of the Nationally
Notifiable Diseases by the Centers for Disease Control.
Clinical Presentation
Legionellae present as two distinct clinical
diseases. The first is Legionnaires' disease, a typical pneumonia with
an incubation period of 2 to 10 days. The mortality rate is as low as 20
% for healthy individuals and as high as 75% for the immune compromised
persons. Legionnaires' disease is treated with erythromycin. The second
form of disease presentation is Pontiac Fever. This illness has an
incubation period of 1 to 2 days and is self-limiting with flu-like
symptoms and no reported mortality.
Pathogenesis
Pathogenesis of Legionellae species requires the
organism be phagocytosed into monocytes via complement receptors. Once
inside the monocytes, the bacteria prevent phagosome-lysosome fusion and
proceed to replicate until they lyse the phagosome which leads to
apoptosis of the monocyte and release of the bacteria. Humoral immunity
has little effect and the sensitized T helper (TH1) cells are required
to activate the infected cells. Interferon- gamma is also critical to
the elimination of Legionellae.
|
|
|
 Return to the Bacteriology Section
of Microbiology and Immunology On-line Return to the Bacteriology Section
of Microbiology and Immunology On-line
This page last changed on
Sunday, March 06, 2016
Page maintained by
Richard Hunt
|

 Photomicrograph of Bordetella (Haemophilus) pertussis bacteria using
Gram stain technique. CDC
Photomicrograph of Bordetella (Haemophilus) pertussis bacteria using
Gram stain technique. CDC Binding of pertussis toxin to cell membrane
Binding of pertussis toxin to cell membrane Incidence of H. influenzae non-type b invasive disease among children <5 years of age,
1996. CDC/Barbara Rice ber2@cdc.gov
Incidence of H. influenzae non-type b invasive disease among children <5 years of age,
1996. CDC/Barbara Rice ber2@cdc.gov  Haemophilus influenzae - coccobacillus prokaryote (dividing); causes meningitis in children, pneumonia,
epiglottitis, laryngitis, conjunctivitis, neonatal infection, otitis media (middle ear infection) and sinusitis in adults
(SEM x 64,000)
©
Dennis Kunkel Microscopy, Inc.
Used with permission
Haemophilus influenzae - coccobacillus prokaryote (dividing); causes meningitis in children, pneumonia,
epiglottitis, laryngitis, conjunctivitis, neonatal infection, otitis media (middle ear infection) and sinusitis in adults
(SEM x 64,000)
©
Dennis Kunkel Microscopy, Inc.
Used with permission  Clinical symptoms of infection by Haemophilus
Clinical symptoms of infection by Haemophilus
 Countries implementing routine childhood Hib immunization © WHO
Countries implementing routine childhood Hib immunization © WHO Legionella pneumophila multiplying inside a cultured
cell. Multiple intracellular bacilli, including dividing bacilli, are visible in longitudinal and cross section. Transmission electron micrograph.
CDC/Dr. Edwin P. Ewing, Jr.
Legionella pneumophila multiplying inside a cultured
cell. Multiple intracellular bacilli, including dividing bacilli, are visible in longitudinal and cross section. Transmission electron micrograph.
CDC/Dr. Edwin P. Ewing, Jr.








