|
x |
x |
 |
 |
|
INFECTIOUS
DISEASE |
BACTERIOLOGY |
IMMUNOLOGY |
MYCOLOGY |
PARASITOLOGY |
VIROLOGY |
|
|
PARASITOLOGY - CHAPTER TWO
BLOOD AND TISSUE PROTOZOA
PART 1
TRYPANOSOMIASIS AND
LEISHMANIASIS
Dr
Abdul
Ghaffar
Professor Emeritus
University of South Carolina
|
|
|
|
|
Let us know what you think
FEEDBACK |
|
SEARCH |
|
|

 |
|
|
CHAPTER TWO
BLOOD AND TISSUE PROTOZOA
SECTIONS
|
|
|
Blood protozoa of
major clinical significance include members of genera:
-
Trypanosoma (T. brucei and
T. cruzi)
-
Leishmania (L. donovani,
L. tropica and L.
braziliensis)
-
Plasmodium (P. falciparum,
P. ovale, P. malariae and
P. vivax)
-
Toxoplasma (T. gondii)
-
Babesia (B. microti)
TRYPANOSOMIASIS
African
trypanosomiasis (Sleeping sickness)
Etiology
There are
two clinical forms of African trypanosomiasis:
-
A slowly developing
(chronic) disease,
West African Sleeping Sickness, caused by Trypanosoma brucei
gambiense
-
A rapidly progressing
(acute) disease,
East African Sleeping Sickness, caused by T. brucei
rhodesiense.
Epidemiology
T. b.
gambiense is found in the western and central regions of Africa, whereas
T. b. rhodesiense is restricted to the eastern third of the continent
(figure 2E). Most cases of sleeping sickness (98%) are the chronic West
African form but the number of new cases have fallen in recent years from
27,862 in 1999 to 6,228 in 2013 (78% reduction). At the same time, the
number of new cases of the acute East African form has fallen from 619 to 86
over the same time period (86% fall).
Most East African Sleeping Sickness
occurs in 13 countries with the highest incidence in Zambia, Malawi, Uganda and Tanzania.
Cases of West African Sleeping Sickness are documented annually
in 24 countries with most in The Central African Republic, The Democratic
Republic of the Congo, northern Uganda, Chad, Angola and Sudan. Thirty five million people and 25
million cattle are at risk. Regional epidemics of the disease have been the
cause of major
health and economic disasters.
Occasionally, a traveler to
endemic counties contracts Sleeping Sickness. About one case of East African
Sleeping Sickness is imported into the United States each year, usually in
someone who has recently travelled to the region. In the case, of West
African Sleeping Sickness, most infections diagnosed in the United States
are in people who have immigrated from an endemic region. These are very
rare.
Vector and Reservoir
In both West African and East African Sleeping Sickness, the vector is
the Tsetse Fly (Glossina sp) and both sexes of the fly can transmit
the parasite in their saliva. In endemic areas, however, only a few flies
are carrieers. The animal reservoir for T. b.
gambiense is other humans but domestic animals can also carry the parasite.
The reservoirs for T. b. rhodesiense are wild animals and cattle.
Very occasionally, an unborn
baby may be infected from an infected mother. It is also possible that
people have been very rarely infected as a result of blood transfusions.
Morphology
T. b.
gambiense and T. b. rhodesiense are similar in appearance: The
organism measures 10 - 30 micrometers x 1-3 micrometers. It has a single central nucleus and a single flagellum originating at the kinetoplast and
joined to the body by an undulating membrane (Figure 2A-D). The outer surface of
the organism is densely coated with a layer of glycoprotein, the variable
surface glycoprotein (VSG).
|
|
TEACHING
OBJECTIVES
Epidemiology,
morbidity and mortality
Morphology of the organism
Life
cycle, hosts and vectors
Disease,
symptoms, pathogenesis and site
Diagnosis
Prevention
and control
|
|

Incidence of T. brucei gambiense sleeping sickness 2013
WHO

Incidence of T. brucei rhodesiense sleeping sickness 2013
WHO |
|
|
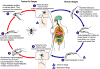 Figure 1A
Figure 1A
During a blood meal on the mammalian host, an infected tsetse fly
(genus Glossina) injects metacyclic trypomastigotes into skin tissue.
The parasites enter the lymphatic system and pass into the bloodstream
 .
Inside the host, they transform into bloodstream trypomastigotes .
Inside the host, they transform into bloodstream trypomastigotes
 ,
are carried to other sites throughout the body, reach other blood fluids (e.g.,
lymph, spinal fluid), and continue the replication by binary fission ,
are carried to other sites throughout the body, reach other blood fluids (e.g.,
lymph, spinal fluid), and continue the replication by binary fission
 .
The entire life cycle of African Trypanosomes is represented by extracellular
stages. The tsetse fly becomes infected with bloodstream trypomastigotes
when taking a blood meal on an infected mammalian host ( .
The entire life cycle of African Trypanosomes is represented by extracellular
stages. The tsetse fly becomes infected with bloodstream trypomastigotes
when taking a blood meal on an infected mammalian host ( , ,
 ). In the fly’s midgut, the
parasites transform into procyclic trypomastigotes, multiply by binary fission ). In the fly’s midgut, the
parasites transform into procyclic trypomastigotes, multiply by binary fission
 ,
leave the midgut, and transform into epimastigotes ,
leave the midgut, and transform into epimastigotes
 .
The epimastigotes reach the fly’s salivary glands and continue multiplication
by binary fission .
The epimastigotes reach the fly’s salivary glands and continue multiplication
by binary fission  . The cycle
in the fly takes approximately 3 weeks. Humans are the main reservoir for Trypanosoma
brucei gambiense, but this species can also be found in animals. Wild
game animals are the main reservoir of T. b. rhodesiense. . The cycle
in the fly takes approximately 3 weeks. Humans are the main reservoir for Trypanosoma
brucei gambiense, but this species can also be found in animals. Wild
game animals are the main reservoir of T. b. rhodesiense.
CDC DPDx Parasite Image Library
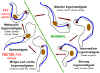 Figure 1B
Figure 1B
Forms of Trypansoma brucei observed in the tsetse
fly and in the human blood stream
T. brucei is transmitted by tsetse flies of the genus Glossina.
Parasites are ingested by the fly when it takes a blood meal on an infected
mammal. The parasites multiply in the fly, going through several developmental
stages in the insect gut and salivary glands (procyclic trypanosomes,
epimastigotes, metacyclic trypanosomes). The cycle in the fly takes
approximately 3 weeks. When the fly bites another mammal, metacyclic
trypanosomes are inoculated, and multiply in the host's blood and extracellular
fluids such as spinal fluid. Humans are the main reservoir for T. b. gambiense,
but this species can also be found in animals. Wild game animals are the main
reservoir of T. b. rhodesiense.
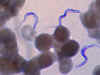
 Figure 2A
Figure 2A
Two areas from a blood smear from a patient with African trypanosomiasis. Thin blood smear stained with Giemsa. Typical
trypomastigote stages (the only stages found in patients), with a
posterior kinetoplast, a centrally located nucleus, an undulating
membrane, and an anterior flagellum. The two Trypanosoma brucei species
that cause human trypanosomiasis, T. b. gambiense and
T. b. rhodesiense, are undistinguishable morphologically. The
trypanosomes length range is 14-33 µm
CDC DPDx Parasite Image Library
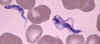 Figure 2B
Figure 2B
Blood smear from a patient (a U.S. traveler) with Trypanosoma
brucei rhodesiense. A dividing parasite is seen at the right. Dividing
forms are seen in African trypanosomiasis, but not in American
trypanosomiasis (Chagas' disease)
CDC DPDx Parasite Image Library
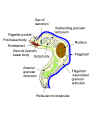 Figure 2D Figure 2D
Structure of Trypanosoma brucei
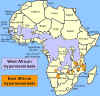 Figure 2E
Figure 2E
Distribution of West African or Gambian Sleeping Sickness and East
African or Rhodesian Sleeping Sickness
 Figure 2C Figure 2C
Blood smear from a patient with Trypanosoma brucei gambiense.
CDC -
Image contributed by Pr. J. Le Bras, Hôpital Bichat - Claude Bernard, Paris,
France.
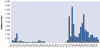 Figure 2F Reported number of cases of African
trypanosomiasis
Figure 2F Reported number of cases of African
trypanosomiasis
in Uganda, 1939-1998 WHO
Between 1962 and 1975, no cases were reported. Increased reporting
during 1977 to 1983 reflected an epidemic of rhodesiense sleeping
sickness in Busuga (south-eastern Uganda). However the increases shown
between 1986 and 1992 corresponded to both the resumption of systematic
population screening for gambiense sleeping sickness in the western part
of the country and to a resurgence of rhodesiense sleeping sickness in
Busuga.
|
| |
 Figure 3 Tsetse fly. The vector of African trypanosomiasis
© OhioState University, College of Biology
Figure 3 Tsetse fly. The vector of African trypanosomiasis
© OhioState University, College of Biology |
Life cycle
The
infective, metacyclic form of the trypanosome is injected into the primary host
during a bite by the vector, the tsetse fly (figure 3). The organism transforms
into a dividing trypanosomal (trypomastigote) blood form (figure 1B) as it enters the
draining lymphatic and blood stream. The trypanosomal form enters the vector
during the blood meal and travels through the alimentary canal to the salivary
gland where it proliferates as the crithidial form (epimastigote) and matures to
infectious metacyclic forms (Figure 1B). Trypomastigotes can traverse the walls
of blood and lymph capillaries into the connective tissues and, at a later
stage, cross the choroid plexus into the brain and cerebrospinal fluid. The
organism can be transmitted through blood transfusion.
|
| |
Symptoms
The clinical
features of Gambian and Rhodesian disease are the same, however they vary in
severity and duration. Rhodesian disease progresses more rapidly and the
symptoms are often more pronounced. The symptoms of the two diseases are also
more pronounced in Caucasians than in the local African population.
Classically, the progression of African trypanosomiasis can be divided into
three stages: the bite reaction (chancre), parasitemia (blood and lymphoid
tissues), and CNS stage.
Bite reaction
A
non-pustular, painful, itchy chancre (Figure 4 A and B) forms 1-3 weeks after the
bite and lasts 1-2 weeks. It leaves no scar.
Parasitemia
Parasitemia and lymph node invasion is marked by attacks of fever which
starts 2-3 weeks after the bite and is accompanied by malaise, lassitude,
insomnia headache and lymphadenopathy and edema (figure 4E). Painful
sensitivity of palms and ulnar region to pressure (Kerandel's sign) may
develop in some Caucasians. Very characteristic of Gambian disease is
visible enlargement of the glands of the posterior cervical region (Winterbottom's
sign) (Figure 4C). Febrile episodes may last few months as in Rhodesian
disease or several years as in Gambian disease. Parasitemia is more
prominent during the acute stage than during the recurrence episodes.
CNS Stage
The
late or CNS stage is marked by changes in character and personality. They
include lack of interest and disinclination to work, avoidance of
acquaintances, morose and melancholic attitude alternating with exaltation,
mental retardation and lethargy, low and tremulous speech, tremors of tongue
and limbs, slow and shuffling gait, altered reflexes, etc. Males become
impotent. There is a slow progressive involvement of cardiac tissue. The
later stages are characterized by drowsiness and uncontrollable urge to
sleep. The terminal stage is marked by wasting and emaciation. Death results
from coma,
intercurrent infection or cardiac failure (figure 5).
In the case of T. b. rhodesiense
disease, death occurs within months of CNS involvement whereas T. b.
gambiense-caused disease is slower and, without treatment, death occurs
within 3 to 7 years.
|
|
Figure
4A
 The partially healed chancre on the arm of a female patient in a ward of a rural clinic.
WHO/TDR/Crump
The partially healed chancre on the arm of a female patient in a ward of a rural clinic.
WHO/TDR/Crump |
 Figure
4B
Figure
4B
The leg of a teenage girl who has sleeping sickness, showing the chancre at the site of the tsetse fly bite
WHO/TDR/Kuzoe
 Figure
4C
Figure
4C
Winterbottoms sign CDC
DPDx Parasite Image Library
 Figure
4D
Figure
4D
Neurological complications can occur as a result of infection and, as seen here, patients may be immobilised for their own safety.
WHO/TDR/Kuzoe
 Figure
4E
Figure
4E
A male sleeping sickness patient with myxoedema.
WHO/TDR/Kuzoe
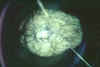 Figure 5A
Figure 5A
The damaged brain of a patient who had died from African trypanosomiasis (or sleeping sickness).
WHO/TDR/Kuzoe
 Figure 5B
Figure 5B
A young boy with advanced African trypanosomiasis (or sleeping sickness) exhibiting marked wasting and skin damage caused as a result of the intense itching which can accompany late-stage disease.
WHO/TDR/Kuzoe
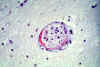 Figure 5C
Figure 5C
Neuropathology of Human African Trypanosomiasis: Acute haemorrhagic leucoencephalopathy
(AHL): This slide shows very delicate fibrinoid necrosis in the wall of a small artery in the thalamus.
Produced by the Dept. of
Neuropathology, Southern General Hospital,
Glasgow).
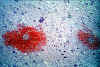 Figure 5D
Figure 5D
Neuropathology of Human African Trypanosomiasis: Acute haemorrhagic
leucoencephalopathy: This slide shows the foci of haemorrhage around small blood vessels.
Produced by the Dept. of
Neuropathology, Southern General Hospital, Glasgow).
|
|
|
| |
The clinical features
of Rhodesian disease are similar but briefer and more acute. The acuteness and
severity of disease do not allow typical sleeping sickness. Death is due to
cardiac failure within 6-9 months.
Pathology and
Immunology
An exact pathogenesis of
sleeping sickness is not known, although
immune complexes and inflammation have been suspected to be the mechanism of
damage to tissues. The immune response against the organism does help to eliminate the
parasite but it is not protective, since the parasite has a unique ability of
altering its surface antigens, the Variable Surface Glycoproteins (VSGs) - see the chapter on
Molecular
Biology of Trypanosomes. Consequently, there is a cyclic fluctuation in
the number of parasites in blood and lymphatic fluids and each wave of parasite
represents a different antigenic variant. The parasite causes polyclonal
expansion of B lymphocytes and plasma cells and an increase in total IgM
concentration. It stimulates the reticuloendothelial function. It also causes
severe depression of cell mediated and humoral immunity to other antigens.
Diagnosis
Detection
of parasite by microscopy in the bloodstream, lymph secretions and enlarged lymph node aspirate
provides a definitive diagnosis in early (acute) stages. Classically, a lymph
node (posterior cervical node) aspirate is used as it may be difficult to
detect a low
parasitemia in the blood. The parasite in blood can
be concentrated by centrifugation or by the use of anionic support media.
Cerebrospinal fluid must always be examined for organisms. Immuno-serology (enzyme-linked immune assay, immunofluorescence) may
be indicative but does not provide definite diagnosis.
Treatment and
Control
The blood stage of African trypanosomiasis can be treated with reasonable
success according to the stage that the disease has reached. Pentamidine isethionate
is used for first stage T. b. gambiense infection. Other drugs
available for use are
suramin,
melarsoprol, eflornithine or nifurtimox. Suramin has been reported
also to be effective in prophylaxis although they may mask early infection and
thus increase the risk of CNS disease. Cases with CNS involvement should be
treated with melarsoprol, an organic arsenic compound; however this drug has
been linked to fatal encephalopathy.
The most effective
means of prevention is to avoid contact with tsetse flies. Vector eradication is
usually impractical due to the vast area involved. Immunization has not been effective
due to antigenic variation.
|
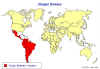 Figure 6 Chaga's disease: Countries in which American trypanosomiasis is endemic.
WHO
Figure 6 Chaga's disease: Countries in which American trypanosomiasis is endemic.
WHO |
American
trypanosomiasis (Chagas' disease)
Etiology
Chagas'
disease is caused by the protozoan hemoflagellate, Trypanosoma cruzi.
Epidemiology
American
trypanosomiasis, also known as Chagas' disease, is scattered irregularly in
Central and South America, stretching from parts of Mexico to Argentina (figure
6). It is estimated that over 8 million people are infected by the parasite and
50 million are at risk. About 50,000 people die each year from the disease.
CDC estimates that there are as
many as 300,000 infected people in the United States and cases
cases have been reported in Texas, California and Maryland. Most of these
infections were acquired in countries of Central and South America where the
disease is endemic and vector-borne cases are very rare..
|
 Figure 7A
Figure 7A
Trypanosoma cruzi, trypomastigote form, in a blood smear (Giemsa stain)
CDC
DPDx Parasite Image Library |
Morphology
Depending
on its host environment, the organism occurs in three different forms (Figure 7
and 9B).
- The trypanosomal (trypomastigote) form (figure 7A), found in mammalian blood, is 15 to 20
microns long and morphologically similar to African trypanosomes.
- The crithidial
(epimastigote) form (figure 7B) is found in the insect intestine.
- The leishmanial
(amastigote)
form (figure 7C), found intracellularly or in pseudocysts in mammalian viscera
(particularly in myocardium and brain), is round or oval in shape, measures 2-4
microns and lacks a prominent flagellum.
|

Figure 7B Trypanosoma cruzi, crithidia.
CDC
DPDx Parasite Image Library |
Life
cycle
The
organism is transmitted to mammalian host by many species of kissing or
triatomine (riduvid)
bug (figure 8), most prominently by Triatoma infestans, Triatoma sordida,
Panstrongylus megistus
and Rhodnius prolixus.Transmission takes place during the feeding of the bug
which normally bites in the facial area (hence the name, kissing bug) and has
the habit of defecating during feeding. The metacyclic trypamastigotes, contained
in the fecal material, gain access to the mammalian tissue through the wound
which is often rubbed by the individual that is bitten. Subsequently,
they enter various cells, including macrophages, where they
differentiate into amastigotes and multiply by binary fission. The
amastigotes differentiate into non-replicating trypomastigotes and the
cells rupture to release them into the bloodstream. Additional host
cells, of a variety of types, can become infected and the
trypomastigotes once again form amastigotes inside these cells. Uninfected
insect vectors acquire the organism when they
feed on infected animals or people containing trypomastigotes circulating in
their blood. Inside the alimentary tract of the insect vector, the trypomastigotes
differentiate to form epimastigotes and divide longitudinally in the mid
and hindgut of the insect where they develop into infective metacyclic
trypomastigotes (figure 9C).
Transmission may also occur
between humans by
- Blood
transfusion
- Mother to baby via a transplacental route
- Organ transplantation
- Very rarely via contaminated
food or drink
|

Figure 7C. Trypanosoma cruzi. Leishmanial form CDC
DPDx Parasite Image Library
 Figure 8 Riduvid bug, the vector of American trypanosomiasis
Figure 8 Riduvid bug, the vector of American trypanosomiasis
 Figure 9A Ramana's sign: unilateral
conjunctivitis and orbital edema
Figure 9A Ramana's sign: unilateral
conjunctivitis and orbital edema
 Figure 9B Megacolon in Chaga's disease
Figure 9B Megacolon in Chaga's disease |
More than one hundred mammalian species of wild and domestic animals including cattle, pigs, cats,
dogs, rats, armadillo, raccoon and opossum are naturally infected by T. cruzi
and serve as a reservoir.
Symptoms
Chagas'
disease can be divided into three stages: the primary lesion, the acute stage,
and the chronic stage. The primary lesion, chagoma, appearing at the site of
infection, within a few hours of a bite, consists of a slightly raised, flat
non-purulent erythematous plaque surrounded by a variable area of hard edema. It
is usually found on the face, eyelids, cheek, lips or the conjunctiva, but may
occur on the abdomen or limbs. When the primary chagoma is on the face, there is
an enlargement of the pre- and post- auricular and the submaxillary glands on
the side of the bite. Infection in the eyelid, resulting in a unilateral
conjunctivitis and orbital edema (Ramana's sign) (figure 9A), is the commonest finding.
Acute Stage: The
acute stage appears 7-14 days after infection. It is characterized by
restlessness, sleeplessness, malaise, increasing exhaustion, chills, fever
and bone and muscle pains. Other manifestations of the acute phase are
cervical, axillary and iliac
adenitis,
hepatomegaly,
erythematous rash and
acute
myocarditis. There is a general edematous reaction associated with
lymphadenopathy. Diffuse myocarditis, sometimes accompanied by serious
pericarditis and endocarditis, is very frequent during the initial stage of
the disease. In children, Chagas' disease may cause meningo-encephalitis and coma. Death
occurs in 5-10 percent of infants. Hematologic examination reveals
lymphocytosis and parasitemia.
Chronic Stage: The
acute stage is usually not recognized and often resolves with little or no
immediate damage and the infected host remains an asymptomatic carrier. An
unknown proportion (guessed at 10-20%) of victims develop a chronic disease.
They alternate between asymptomatic remission periods and relapses
characterized by symptoms seen in the acute phase. Cardiac arrhythmia is
common. The chronic disease results in an abnormal function of the hollow
organs, particularly the heart, esophagus and colon.
The cardiac changes
include myocardial insufficiency, cardiomegaly, disturbances of atrio-ventricular
conduction and the Adams-Stoke syndrome. Disturbances of peristalsis lead to
megaesophagus and megacolon (figure 9B).
|
|
|
Pathology and
Immunology
The pathological effects of acute phase
Chagas' disease largely result from
direct damage to infected cells. In later stages, the destruction of the autonomic
nerve ganglions may be of significance. Immune mechanisms, both cell mediated
and humoral, involving reaction to the organism and to autologous tissues have
been implicated in pathogenesis.
T. cruzi stimulates
both humoral and cell mediated immune responses. Antibody has been shown to lyze
the organism, but rarely causes eradication of the organism, perhaps due to its
intracellular localization. Cell mediated immunity may be of significant value.
While normal macrophages are targeted by the organism for growth, activated
macrophages can kill the organism. Unlike T. brucei, T. cruzi does not alter its
antigenic coat. Antibodies directed against heart and muscle cells have also
been detected in infected patients leading to the supposition that there is an element of autoimmune reaction in the
pathogenesis of Chagas' disease. The infection causes severe depression of both
cell mediated and humoral immune responses. Immunosuppression may be due to
induction of suppressor T-cells and/or overstimulation of macrophages.
Diagnosis
Clinical
diagnosis is usually easy among children in endemic areas. Cardiac dilation,
megacolon and megaesophagus in individuals from endemic areas indicate present
or former infection. Definitive diagnosis requires the demonstration of
trypanosomes by microscopy or biological tests (in the insect or mice). Antibodies
are often detectable by complement fixation or immunofluorescence and provide
presumptive diagnosis.
Treatment and
Control
There is no curative therapy available. Most drugs are either ineffective or
highly toxic. Recently two experimental drugs, Benznidazol and Nifurtimox have
been used with promising results in the acute stage of the disease, however
their side effects limit their prolonged use in chronic cases.
Control measures are
limited to those that reduce contact between the vectors and man. Attempts to
develop a vaccine have not been very successful, although they may be feasible.
|
| |
|
|
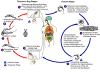 Figure 9C
Figure 9C
An infected triatomine insect vector (or “kissing” bug) takes
a blood meal and releases trypomastigotes in its feces near the site of the bite
wound. Trypomastigotes enter the host through the wound or through intact
mucosal membranes, such as the conjunctiva
 .
Common triatomine vector species for trypanosomiasis belong to the genera Triatoma,
Rhodinius, and Panstrongylus. Inside the host, the
trypomastigotes invade cells, where they differentiate into intracellular
amastigotes .
Common triatomine vector species for trypanosomiasis belong to the genera Triatoma,
Rhodinius, and Panstrongylus. Inside the host, the
trypomastigotes invade cells, where they differentiate into intracellular
amastigotes  . The amastigotes
multiply by binary fission . The amastigotes
multiply by binary fission  and
differentiate into trypomastigotes, and then are released into the circulation
as bloodstream trypomastigotes and
differentiate into trypomastigotes, and then are released into the circulation
as bloodstream trypomastigotes  .
Trypomastigotes infect cells from a variety of tissues and transform into
intracellular amastigotes in new infection sites. Clinical manifestations
can result from this infective cycle. The bloodstream trypomastigotes do
not replicate (different from the African trypanosomes). Replication
resumes only when the parasites enter another cell or are ingested by another
vector. The “kissing” bug becomes infected by feeding on human or
animal blood that contains circulating parasites .
Trypomastigotes infect cells from a variety of tissues and transform into
intracellular amastigotes in new infection sites. Clinical manifestations
can result from this infective cycle. The bloodstream trypomastigotes do
not replicate (different from the African trypanosomes). Replication
resumes only when the parasites enter another cell or are ingested by another
vector. The “kissing” bug becomes infected by feeding on human or
animal blood that contains circulating parasites
 .
The ingested trypomastigotes transform into epimastigotes in the vector’s
midgut .
The ingested trypomastigotes transform into epimastigotes in the vector’s
midgut  . The parasites
multiply and differentiate in the midgut . The parasites
multiply and differentiate in the midgut
 and differentiate into infective metacyclic trypomastigotes in the hindgut
and differentiate into infective metacyclic trypomastigotes in the hindgut
 . .
Trypanosoma cruzi can also be transmitted through blood transfusions,
organ transplantation, transplacentally, and in laboratory accidents.
CDC
DPDx Parasite Image Library
|
|
Guest article
New approaches
for vaccines against a neglected disease – leishmaniasis |
LEISHMANIASIS
Etiology
More than 20
species of Leishmania are pathogenic for man:
-
L. donovani causes visceral
leishmaniasis (Kala-azar, black disease, dumdum fever)
-
L. tropica
(L. t.
major, L. t. minor and L. ethiopica) causes cutaneous leishmaniasis (oriental
sore, Delhi ulcer, Aleppo, Delhi or Baghdad boil)
-
L. braziliensis
(also, L. mexicana and L. peruviana) are etiologic agents of mucocutaneous
leishmaniasis (espundia, Uta, chiclero ulcer)
Epidemiology
Leishmaniasis is prevalent
in more than 90 countries world wide: ranging from south east Asia,
Indo-Pakistan, Mediterranean area of southern Europe, north and central Africa, and south and central
America. A few cases of cutaneous leishmaniasis have been found in the United
States (Texas and Oklahoma). These have been acquired during travel to
endemic areas
The annual number of cases
worldwide have been estimated to be:
-
Cutaneous leishmaniasis:
Between 700,000 and 1.2 million
-
Visceral leishmaniasis:
Between 200,000 and 400,000
Morphology
Amastigote
(leishmanial form) is oval and measures 2-5 microns by 1 - 3 microns (figure
10A-D), whereas the
leptomonad measures 14 - 20 microns by 1.5 - 4 microns, a similar size to trypanosomes
(Figure 10E).
|
|

Incidence of cutaneous Leishmaniasis 2012
WHO

Incidence of visceral Leishmaniasis 2012
WHO |
|
Figure
10 A B C
 A
A
 B
B
 C
C
Leishmania tropica amastigotes from a skin touch preparation. In A, a
still intact macrophage is practically filled with amastigotes, several
of which have clearly visible a nucleus and a kinetoplast (arrows); in
B, amastigotes are being freed from a rupturing macrophage. Patient with
history of travel to Egypt, Africa, and the Middle East. Culture in NNN
medium followed by isoenzyme analysis identified the species as L.
tropica minor. CDC |
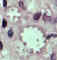 Figure 10D
Figure 10D
Leishmania mexicana mexicana in skin biopsy. Hematoxylin and eosin stain. The amastigotes are lining the wall of two
vacuoles, a typical arrangement. The species identification was derived from culture followed by isoenzyme analysis. 26-year old man from Austin, Texas, with a lesion on his left arm.
CDC DPDx Parasite Image Library
 Figure 10E
Figure 10E
Leishmania donovani, leptomonad forms.
CDC
DPDx Parasite Image Library
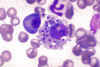 Figure 10G Figure 10G
Bone marrow smear showing Leishmania donovani
parasites in a bone marrow histiocyte from a dog
(Giemsa stain).
CDC/Dr. Francis W. Chandler
 Figure 10I
Figure 10I
Leishmania donovani in bone marrow cell. Smear.
CDC/Dr. L.L. Moore, Jr.
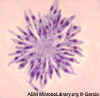 Figure 10 F
Figure 10 F
Giemsa stained
leishmanial promastigotes from a culture in which the bar-shaped kinetoplast in the organism closest to the center of the group "rosette" may be
seen.
©
Lynne S. Garcia,
LSG & Associates, Santa Monica, California
and Microbe Library
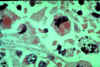 Figure 10H
Figure 10H
Erythrophagocytosis in the liver (H&E X 400)
WHO/TDR/El-Hassan
 Figure 10J
Figure 10J
Periarterial sheath of macrophages of the spleen showing
heavy parasitisation with amastigotes (H&E X 400)
WHO/TDR/El-Hassan
|
| |
Life cycle
The
organism is transmitted by the bite of about 30 species of blood-feeding sand
flies (Phlebotomus) which carry the promastigote in the anterior gut and
pharynx. The parasites gain access to mononuclear phagocytes where they transform into
amastigotes and divide until the infected cell ruptures. The released organisms
infect other cells. The sandfly acquires the organisms during the blood meal;
the amastigotes transform into flagellate promastigotes and multiply in the gut
until the anterior gut and pharynx are packed. Dogs and rodents are
common reservoirs (figure 11F).
Symptoms
Visceral
leishmaniasis (kala-azar, dumdum fever)
L. donovani organisms in visceral
leishmaniasis are rapidly eliminated from the site of infection, hence there
is rarely a local lesion, although minute papules have been described in
children. They are localized and multiply in the mononuclear phagocytic cells
of spleen, liver, lymph nodes, bone marrow, intestinal mucosa and other
organs. One to four months after infection, there is occurrence of fever, with a
daily rise to 102-104 degrees F, accompanied by chills and sweating.
The spleen
and liver progressively become enlarged (figure 11B, C and E). With progression of the diseases,
skin develops hyperpigmented granulomatous areas (kala-azar means black disease).
Chronic disease renders patients susceptible to other infections. Untreated
disease results in death.
|

Figure 11A
Many children suffering from visceral leishmaniasis develop a noticeable thickening, stiffening and darkening of the eyelashes and eyebrows.
WHO/TDR/Crump |
 Figure 11B
Figure 11B
Profile view of a teenage boy suffering from visceral
leishmaniasis. The
boy exhibits splenomegaly, distended abdomen and severe muscle wasting.
WHO/TDR/Kuzoe
 Figure 11C
Figure 11C
A 12-year-old boy suffering from visceral
leishmaniasis. The boy exhibits
splenomegaly and severe muscle wasting.
WHO/TDR/El-Hassan
 Figure 11D
Figure 11D
Jaundiced hands of a visceral leishmaniasis patient.
WHO/TDR/El-Hassan
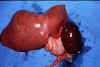 Figure 11E Figure 11E
Enlarged spleen and liver in an autopsy of an infant dying of visceral
leishmaniasis.
WHO/TDR/El-Hassan
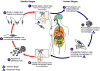 Figure 11F
Figure 11F
Leishmaniasis is transmitted by the bite of female phlebotomine
sandflies. The sandflies inject the infective stage, promastigotes,
during blood meals  .
Promastigotes that reach the puncture wound are phagocytized by
macrophages .
Promastigotes that reach the puncture wound are phagocytized by
macrophages  and transform
into amastigotes and transform
into amastigotes  .
Amastigotes multiply in infected cells and affect different tissues,
depending in part on the Leishmania species .
Amastigotes multiply in infected cells and affect different tissues,
depending in part on the Leishmania species
 .
This originates the clinical manifestations of leishmaniasis.
Sandflies become infected during blood meals on an infected host when
they ingest macrophages infected with amastigotes ( .
This originates the clinical manifestations of leishmaniasis.
Sandflies become infected during blood meals on an infected host when
they ingest macrophages infected with amastigotes ( , ,
 ). In the sandfly's
midgut, the parasites differentiate into promastigotes ). In the sandfly's
midgut, the parasites differentiate into promastigotes
 ,
which multiply and migrate to the proboscis ,
which multiply and migrate to the proboscis
 . .
CDC
DPDx Parasite Image Library
|
|
|
Cutaneous
leishmaniasis (Oriental sore, Delhi ulcer, Baghdad boil)
In cutaneous
leishmaniasis, the organism (L. tropica) multiplies locally, producing of a
papule, 1-2 weeks (or as long as 1-2 months) after the bite. The papule gradually
grows to form a relatively painless ulcer. The center of the ulcer encrusts
while satellite papules develop at the periphery. The ulcer heals in 2-10
months, even if untreated but leaves a disfiguring scar (figure 12). The disease may
disseminate in the case of depressed immune function.
Mucocutaneous
leishmaniasis (espundia, Uta, chiclero)
The initial symptoms of
mucocutaneous leishmaniasis are the same as those of cutaneous leishmaniasis,
except that in this disease the organism can metastasize and the lesions
spread to mucoid (oral, pharyngeal and nasal) tissues and lead to their
destruction and hence sever deformity (figure 12E). The organisms responsible are
L. braziliensis, L. mexicana and L. peruviana.
Pathology
Pathogenesis of leishmaniasis is due to an immune reaction to the organism,
particularly cell mediated immunity. Laboratory examination reveals a marked
leukopenia with relative monocytosis and lymphocytosis, anemia and
thrombocytopenia. IgM and IgG levels are extremely elevated due to both specific
antibodies and polyclonal activation.
Diagnosis
Diagnosis
is based on a history of exposure to sandfies, symptoms and isolation of the
organisms from the lesion aspirate or biopsy, by direct examination or culture.
A skin test (delayed hypersensitivity: Montenegro test) and detection of anti-leishmanial
antibodies by immuno-fluorescence are indicative of exposure.
Treatment and
Control
Sodium stibogluconate (Pentostam) is the drug of choice. Pentamidine isethionate
is used as an alternative. Control measures involve vector control and
avoidance. Immunization has not so far been effective but a
new vaccine
is under investigation.
|
|
Figure
11F |
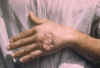 Figure 12A
Figure 12A
Skin ulcer due to leishmaniasis, hand of Central American
adult.
CDC/Dr. D.S. Martin
 Figure
12C Figure
12C
Scar on skin of upper leg representing healed lesion of
leishmaniasis
CDC
 Figure 12D
Figure 12D
Non-healing
cutaneous leishmaniasis lesion on ear lobe
WHO/TDR/El-Hassan
 Figure 12E
Figure 12E
Girl with diffuse muco- cutaneous leishmaniasis of the face
which is responding to treatment
WHO/TDR/El-Hassan
 Figure 12F Figure 12F
Cutaneous leishmaniasis skin lesion. The lesion measured about 1 inch in diameter and was moist with raised borders. There was no drainage;
however, the lesion did appear to be infected.
© Lynne S. Garcia, LSG & Associates and
The
Microbe Library
|
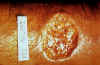
Figure
12B
Crater lesion of leishmaniasis, skin CDC |
|

 |
 Return to the Parasitology Section of Microbiology and Immunology On-line
Return to the Parasitology Section of Microbiology and Immunology On-line
This page last changed on
Tuesday, February 24, 2015
Page maintained by
Richard Hunt
|


 Figure 1A
Figure 1A

 Figure 2A
Figure 2A  Figure 2D
Figure 2D  Figure 2C
Figure 2C  Figure 3 Tsetse fly. The vector of African trypanosomiasis
© OhioState University, College of Biology
Figure 3 Tsetse fly. The vector of African trypanosomiasis
© OhioState University, College of Biology Figure 6 Chaga's disease: Countries in which American trypanosomiasis is endemic.
WHO
Figure 6 Chaga's disease: Countries in which American trypanosomiasis is endemic.
WHO Figure 7A
Figure 7A 

 Figure 9C
Figure 9C Figure 10D
Figure 10D  Figure 10G
Figure 10G
 Figure 10 F
Figure 10 F
 Figure 10J
Figure 10J 
 Figure 11B
Figure 11B  Figure 11D
Figure 11D  Figure 11F
Figure 11F Figure 12A
Figure 12A  Figure 12D
Figure 12D  Figure 12F
Figure 12F




























