|
x |
x |
 |
 |
|
INFECTIOUS
DISEASE |
BACTERIOLOGY |
IMMUNOLOGY |
MYCOLOGY |
PARASITOLOGY |
VIROLOGY |
|
|
PARASITOLOGY - CHAPTER TWO
BLOOD AND TISSUE PROTOZOA
PART 3
OTHER PROTOZOA
Dr
Abdul
Ghaffar
Professor Emeritus
University of South Carolina
|
|
|
Let us know what you think
FEEDBACK |
|
SEARCH |
|
|

 |
CHAPTER TWO
BLOOD AND TISSUE PROTOZOA
SECTIONS
|

Figure 21A
Babesia microti infection, Giemsa-stained thin smear. The organisms resemble
Plasmodium falciparum; however Babesia parasites present several
distinguishing features: they vary more in shape and in size; and they do not produce pigment. A 67 year old woman, status
post-splenectomy, infection probably acquired in Long island (New York)
CDC |
BABESIOSIS
Babesiosis, like malaria, is an infection
of erythrocytes. It is spread by ticks.
Etiology
Babesia
microti is the most important member of the genus that infects man, although
a few cases of infection by Babesia sp. have been detected.
Epidemiology
In the United States, infections are usually seen in the northeast
and the upper mid-west (figure 21E) during the summer months (figure
21F) when ticks are more likely to come in contact with
humans. In 2012, there were 911 reported cases of babeosis (figure
21G). Patients had a median age of 62 (figure 21H) and two thirds
were male, probably reflecting the fact that men are more likely to
come in contact with Ixodid ticks.
Morphology
The
trophozoite is very similar to the ring form of the Plasmodium species (figure
21A and B).
|
|
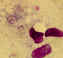
 Figure 21B
Figure 21B
Infection with Babesia. Giemsa-stained thin smears. Note the tetrad (left side of the image), a dividing form pathognomonic for
Babesia. A 6 year old girl, status post splenectomy for hereditary
spherocytosis, infection acquired in the US.
CDC
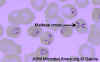 Figure 21C
Figure 21C
Thin blood film of B.
microti ring forms with a typical Maltese Cross (four rings in cross
formation).
© MicrobeLibrary and Lynne Garcia,
LSG & Associates |
Life cycle
The
organism (sporozoite) is transmitted by a tick (Ixodes scapularis) and enters the red cell where it
undergoes mitosis and the organisms (merozoite) are released to infect other red
cells. Ticks acquire the organism during feeding on an infected
individual. In the tick, the organism divides sexually in the gut and migrates
into the salivary gland (figure 21D).
Babesiosis has also be spread by blood transfusion and from other to
fetus.Symptoms
Infections are often asymptomatic and in others there are flu-like
symptoms:
-
fever
-
malaise
-
chills sweats
-
general aches and
pains
However, the destruction
of erythrocytes can lead to:
-
hemolytic anemia
-
jaundice
-
hepatomegaly
These occur usually
1 to 2 weeks after infection. Although usually not severe, babeosis can be
life-threatening as a result of additional complications including
thrombocytopenia, low blood pressure, disseminated intravascular
coagulation (consumptive coagulopathy) leading to thromboses, and
organ collapse. This can be fatal, especially in immunosuppressed
patients, the elderly and those that have undergone
splenectomy.
Diagnosis
Diagnosis
is based on symptoms, patient history and detection of intraerythrocytic
parasite in the blood (figure 21B,D) or transfer of blood in normal hamsters which can be
heavily parasitized.
Treatment and
Control
Drugs of choice are clindamycin combined with quinine or
atovaquone combined with azithromycin.
The patient may recover
spontaneously. One should avoid tick exposure and, if bitten, remove the tick from
the skin
immediately.
|
| |
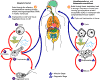
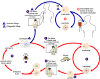 Figure 21D
Figure 21D
The
Babesia microti life cycle involves two hosts, which includes a
rodent, primarily the white-footed mouse, Peromyscus leucopus.
During a blood meal, a Babesia-infected tick introduces
sporozoites into the mouse host
 .
Sporozoites enter erythrocytes and undergo asexual reproduction
(budding) .
Sporozoites enter erythrocytes and undergo asexual reproduction
(budding)  . In the
blood, some parasites differentiate into male and female gametes
although these cannot be distinguished at the light microscope level . In the
blood, some parasites differentiate into male and female gametes
although these cannot be distinguished at the light microscope level
 .
The definitive host is a tick, in this case the deer tick, Ixodes
dammini (I. scapularis). Once ingested by an
appropriate tick .
The definitive host is a tick, in this case the deer tick, Ixodes
dammini (I. scapularis). Once ingested by an
appropriate tick  , gametes
unite and undergo a sporogonic cycle resulting in sporozoites , gametes
unite and undergo a sporogonic cycle resulting in sporozoites
 .
Transovarial transmission (also known as vertical, or hereditary,
transmission) has been documented for “large” Babesia spp.
but not for the “small” babesiae, such as B. microti .
Transovarial transmission (also known as vertical, or hereditary,
transmission) has been documented for “large” Babesia spp.
but not for the “small” babesiae, such as B. microti
 .
Humans enter the cycle when bitten by infected ticks. During a
blood meal, a Babesia-infected tick introduces sporozoites into
the human host .
Humans enter the cycle when bitten by infected ticks. During a
blood meal, a Babesia-infected tick introduces sporozoites into
the human host  .
Sporozoites enter erythrocytes .
Sporozoites enter erythrocytes
 and undergo asexual replication (budding)
and undergo asexual replication (budding)
 .
Multiplication of the blood stage parasites is responsible for the
clinical manifestations of the disease. Humans are, for all
practical purposes, dead-end hosts and there is probably little, if any,
subsequent transmission that occurs from ticks feeding on infected
persons. However, human to human transmission is well recognized
to occur through blood transfusions .
Multiplication of the blood stage parasites is responsible for the
clinical manifestations of the disease. Humans are, for all
practical purposes, dead-end hosts and there is probably little, if any,
subsequent transmission that occurs from ticks feeding on infected
persons. However, human to human transmission is well recognized
to occur through blood transfusions
 .
Note:
Deer are the hosts upon which the adult ticks feed and are indirectly
part of the Babesia cycle as they influence the tick population.
When deer populations increase, the tick population also increases, thus
heightening the potential for transmission. .
Note:
Deer are the hosts upon which the adult ticks feed and are indirectly
part of the Babesia cycle as they influence the tick population.
When deer populations increase, the tick population also increases, thus
heightening the potential for transmission.
CDC
DPDx Parasite Image Library
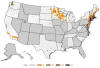 Figure 21E
Figure 21E
Number of reported cases of babesiosis, by county of residence — 27
states, 2013
CDC
 Figure 21F
Figure 21F
Number of reported cases of babesiosis, by month of symptom onset — 2013
CDC
 Figure 21G
Figure 21G
Number of reported cases of babesiosis, by year
CDC
 Figure 21H
Figure 21H
Number of reported cases of babesiosis, by age group — 2013
CDC
|
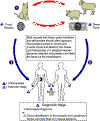
Figure
22 Members
of the cat family (Felidae) are the only known definitive hosts for the
sexual stages of T. gondii and thus are the main reservoirs of
infection. Cats become infected with T. gondii by
carnivorism (1).
After tissue cysts or oocysts are ingested by the cat, viable organisms
are released and invade epithelial cells of the small intestine where
they undergo an asexual followed by a sexual cycle and then form oocysts,
which are then excreted. The unsporulated oocyst takes 1 to 5 days
after excretion to sporulate (become infective). Although cats
shed oocysts for only 1 to 2 weeks, large numbers may be shed.
Oocysts can survive in the environment for several months and are
remarkably resistant to disinfectants, freezing, and drying, but are
killed by heating to 70°C for 10 minutes.
Human infection may be acquired in several ways: A) ingestion of
undercooked infected meat containing Toxoplasma cysts (2);
B) ingestion of the oocyst from fecally contaminated hands or food (3);
C) organ transplantation or blood transfusion; D) transplacental
transmission; E) accidental inoculation of tachyzoites. The
parasites form tissue cysts, most commonly in skeletal muscle,
myocardium, and brain; these cysts may remain throughout the life of the
host.
CDC DPDx Parasite Image Library |
TOXOPLASMOSIS
Etiology
Toxoplasma
gondii is the organism responsible for toxoplasmosis
Epidemiology
Toxoplasma has worldwide distribution and 20%-75% of the population is
seropositive without any symptomatic episode.
In the United States, 22.5% of the population is seropositive. However, the infection poses a
serious threat in immunosuppressed individuals and pregnant females.
The most common routes for human
infection are:
Toxoplasma may also be spread
congenitally (from a mother with no symptoms) and rarely via blood
transfusions and organ transplants.
Morphology
The
intracellular parasites (tachyzoite) are 3x6 microns, pear-shaped organisms that
are enclosed in a parasite membrane to form a cyst measuring 10-100 microns in
size. Cysts in cat feces (oocysts) are 10-13 microns in diameter (figure 22).
Life cycle
The
natural life cycle of T. gondii occurs in cats and small rodents, although the
parasite can grow in the organs (brain, eye, skeletal muscle, etc.) of any
mammal or birds (Figure 22). Cats gets infected by ingestion of cysts in flesh.
Decystation occurs in the small intestine, and the organisms penetrate the
submucosal epithelial cells where they undergo several generations of mitosis,
finally resulting in the development of micro- (male) and macro- (female)
gametocytes. Fertilized macro-gametocytes develop into oocysts that are
discharged into the gut lumen and excreted. Oocysts sporulate in the warm
environment and are infectious to a variety of animals including rodents and
man. Sporozoites released from the oocyst in the small intestine penetrate the
intestinal mucosa and find their way into macrophages where they divide very
rapidly (hence the name tachyzoites) (figure 23) and form a cyst which may occupy the whole
cell. The infected cells ultimately burst and release the tachyzoites to enter
other cells, including muscle and nerve cells, where they are protected from the
host immune system and multiply slowly (bradyzoites). These cysts are infectious
to carnivores (including man). Unless man is eaten by a cat, it is a dead-end
host.
Symptoms
Although
Toxoplasma infection is common, it rarely produces symptoms in normal
individuals and when symptoms do occur, they are flu-like and sometimes
associated with lymphadenopathy. Serious consequences are limited to pregnant women and immunodeficient hosts.
Congenital infections
These occur in about 1 to 5 per 1000
pregnancies of which 5 to 10% result in miscarriage and 8 to 10% result in serious brain
and eye damage to the fetus. 10 to 13% of the babies will have visual handicaps.
Although 58 to 70% of infected women will give birth to a normal offspring, a small proportion
of babies will develop active retino-chorditis or mental retardation in
childhood or young adulthood. Eye lesions are often not identified at birth but
are found in 20 to 80% of infected patients by adulthood. In the United
States fewer than 2% of patients develop eye lesions.
Immunocompromized
patients
In immunocompromized individuals, infection results in generalized parasitemia
involvement of brain, liver lung and other organs, and often death.
Immunology
Both
humoral and cell mediated immune responses are stimulated in normal individuals.
Cell-mediated immunity is protective and humoral response is of diagnostic value.
Diagnosis
Suspected
toxoplasmosis can be confirmed by isolation of the organism from tonsil or lymph
gland biopsy and by serologic testing.
Treatment
Acute
infections benefit from pyrimethamine or sulphadiazine. Spiramycin is a
successful alternative. Pregnant women are advised to avoid cat litter and to handle
uncooked and undercooked meat carefully.
|
|
Figure
23
|
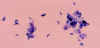 Figure 23A Figure 23A
Toxoplasma gondii in the bronchoalveolar lavage (BAL) material from an HIV infected patient. Numerous
trophozoites (tachyzoites) can be seen, which are typically crescent shaped with a
prominent, centrally placed nucleus. Most of the tachyzoites are free, some are still associated with bronchopulmonary cells.
CDC 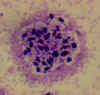 Figure 23B Figure 23B
Toxoplasma gondii in tissue from a cat.
CDC
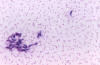 Figure 23C
Figure 23C
Toxoplasma gondii in mouse
ascitic fluid. Smear
CDC
|
| |
PNEUMOCYSTIS
PNEUMONIA
Pneumocystis
jiroveci (formerly known as Pneumocystis carinii)
Pneumocystis
jiroveci was formerly thought to be a protozoan but is now known to be a
fungus. It is included here because pneumocystis pneumonia is often
described as an opportunistic parasitic disease.
Pneumocystis pneumonia
is an infection
of immunosuppressed individuals and is particularly seen in AIDS patients. In
the United States, about 10% of AIDS patients and about 1% of solid
organ transplant recipients are infected.
The organism is pleomorphic, exhibiting, at various stages of its life cycle: 1-2 micron sporozoites, 4-5
micron trophozoites and 6-8 micron
cysts. It spreads from person to person in cough droplets. Infection in
immunosuppressed individuals results in interstitial pneumonia characterized by
thickened alveolar septum infiltrated with lymphocytes and plasma cells.
Pneumonia is associated with fever,
tachypnea, hypoxia,
cyanosis and asphyxia.
Diagnosis is based on isolation of organisms from affected lungs.
Trimethoprim-sulphamethoxazole is the treatment of choice (figure 24).
The mortality rate for P. jiroveci infections is 5 to 40%
when treated and near 100% when untreated.
|
|
|
|
|
 Figure 24A
Figure 24A
Pneumocystis jiroveci trophozoites in broncho-alveolar lavage (BAL) material. Giemsa stain. The trophozoite are small (size: 1-5 µm), and only their nuclei, stained purple, are visible (arrows). AIDS
patient seen in Atlanta, Georgia
CDC
 Figure
24 B and C Figure
24 B and C
Pneumocystis jiroveci cysts
B. 3 cysts in bronchoalveolar material, Giemsa stain; the rounded cysts (size 4-7 µm) contain 6-8 intracystic bodies, whose nuclei are stained by
Giemsa; the walls of the cysts
are not stained; note the presence of several smaller, isolated
trophozoites.
C. cysts in lung tissue, silver stain; the walls of the cysts are stained black; the intracystic bodies are not visible with this stain; baby
who died with pneumonia in California. CDC
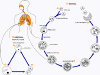 Figure 24D Figure 24D
This
is a generalized life cycle proposed by John J. Ruffolo, Ph.D. (Cushion,
MT, 1988) for the various species of Pneumocystis. These
fungi are found in the lungs of mammals where they reside without
causing overt infection until the host's immune system becomes
debilitated. Then, an oftentimes lethal pneumonia can result.
Asexual phase: trophic forms  replicate by mitosis
replicate by mitosis  to to
 .
Sexual phase: haploid trophic forms conjugate .
Sexual phase: haploid trophic forms conjugate
 and produce a zygote or sporocyte (early cyst)
and produce a zygote or sporocyte (early cyst)
 .
The zygote undergoes meiosis and subsequent mitosis to produce
eight haploid nuclei (late phase cyst) .
The zygote undergoes meiosis and subsequent mitosis to produce
eight haploid nuclei (late phase cyst)
 .
Spores exhibit different shapes (such as, spherical and elongated
forms). It is postulated that elongation of the spores
precedes release from the spore case. It is believed that the
release occurs through a rent in the cell wall. After release, the
empty spore case usually collapses, but retains some residual cytoplasm .
Spores exhibit different shapes (such as, spherical and elongated
forms). It is postulated that elongation of the spores
precedes release from the spore case. It is believed that the
release occurs through a rent in the cell wall. After release, the
empty spore case usually collapses, but retains some residual cytoplasm
 .
A trophic stage, where the organisms probably multiply by binary fission
is also recognized to exist. The organism causes disease in
immunosuppressed individuals. .
A trophic stage, where the organisms probably multiply by binary fission
is also recognized to exist. The organism causes disease in
immunosuppressed individuals.
Pneumocystis
stages were reproduced from a drawing by Dr. John J. Ruffolo, South
Dakota State University, USA. Reproduced by permission of Arnold
and Dr. Ruffolo. Thanks to Dr. Melanie T. Cushion for her comments on
the life cycle text. References:
Ruffolo JJ. Pneumocystis carinii Cell Structure. In:
Walzer, PD, editor. Pneumocystis carinii Pneumonia. 2nd
ed. Marcel Dekker; 1994. p. 25-43.
Cushion MT, Ruffolo JJ, Walzer PD. Analysis of the developmental
stages of Pneumocystis carinii in vitro. Lab Invest
1988;58:324-331.
CDC DPDx Parasite Image Library |
| |
| |
FACULTATIVE
PARASITIC PROTOZOA
These are free-living
amebae that occasionally cause serious human disease. They are of particular
significance in immunocompromised hosts.
Naegleria
fowleri
This organism causes a rare
disease. It is a flagellate that may inhabit warm waters (spas, warm springs, heated
swimming pools, etc.) and gain access via the nasal passage to the brain and
cause primary amebic
meningoencephalitis which is almost always fatal (figure 25). Only three
people out of 132 have survived primary amebic meningoencephalitis in the
last 50 years.
Naegleria fowleri is
sometimes call "the brain eating ameba". Although Naegleria can be
found in contaminated tap water, human infection does not result from
drinking the water.
Epidemiology
In the United States,
infections are rare with only 34 cases between 2004 and 2013. These
resulted from
-
Contaminated
recreational water (3o cases)
-
Nasal irrigation with
contaminated tap water (3 cases)
-
Contaminated tap water
on a backyard slide (1 case)
Symptoms
One to seven days after nasal exposure, the patient suffers:
-
Sever headache
-
Nausea/vomiting
-
Fever
As the meningoencephalitis
develops, patients then experience:
Treatment
There is an investigational drug, miltefosine, that may show promise. In
2013, two children survived an infection. One started treatment 36 hours
after onset of treatment. She was treated with therapeutic hypothermia
and miltefosine. She made a complete recovery. Another child did not
receive hypothermia and was treated later after the onset of symptoms.
He did receive miltefosine. He suffered permanent brain damage.
|
|
|
 Figure 25 A
Figure 25 A
Naegleria fowleri trophozoites, cultured from cerebrospinal fluid. These cells have characteristically large nuclei, with a large, dark staining
karyosome. The amebae are very active and extend and retract pseudopods. Trichrome stain. From a patient who died from primary amebic meningoencephalitis in Virginia.
CDC
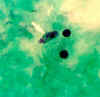 Figure 25B
Figure 25B
Naegleria fowleri trophozoite in spinal fluid. Trichrome stain. Note the typically large karyosome and the monopodial locomotion. Image contributed by Texas
SHD.
CDC
 Figure 25C Figure 25C
Histopathology of amebic meningoencephalitis due to
Naegleria
fowleri. Direct fluorescent antibody stain.
CDC/Dr. Govinda S. Visvesvara gsv1@cdc.gov
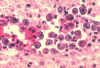 Figure 25D
Figure 25D
Histopathology of Naegleria infection of brain.
CDC
 Figure
25E
Figure
25E
Free-living
amebae belonging to the genera Acanthamoeba, Balamuthia,
and Naegleria are important causes of disease in humans and
animals. Naegleria fowleri produces an acute, and usually
lethal, central nervous system (CNS) disease called primary amebic
meingoencephalitis (PAM). N. fowleri has three stages,
cysts  , trophozoites , trophozoites
 ,
and flagellated forms ,
and flagellated forms  , in
its life cycle. The trophozoites replicate by promitosis (nuclear
membrane remains intact) , in
its life cycle. The trophozoites replicate by promitosis (nuclear
membrane remains intact)  .
Naegleria fowleri is found in fresh water, soil, thermal
discharges of power plants, heated swimming pools, hydrotherapy and
medicinal pools, aquariums, and sewage. Trophozoites can turn into
temporary flagellated forms which usually revert back to the trophozoite
stage. Trophozoites infect humans or animals by entering the
olfactory neuroepithelium .
Naegleria fowleri is found in fresh water, soil, thermal
discharges of power plants, heated swimming pools, hydrotherapy and
medicinal pools, aquariums, and sewage. Trophozoites can turn into
temporary flagellated forms which usually revert back to the trophozoite
stage. Trophozoites infect humans or animals by entering the
olfactory neuroepithelium  and
reaching the brain. N. fowleri trophozoites are found in
cerebrospinal fluid (CSF) and tissue, while flagellated forms are found
in CSF. and
reaching the brain. N. fowleri trophozoites are found in
cerebrospinal fluid (CSF) and tissue, while flagellated forms are found
in CSF.
Acanthamoeba spp. and Balamuthia mandrillaris are
opportunistic free-living amebae capable of causing granulomatous amebic
encephalitis (GAE) in individuals with compromised immune systems.
Acanthamoeba spp. have been found in soil; fresh, brackish, and
sea water; sewage; swimming pools; contact lens equipment; medicinal
pools; dental treatment units; dialysis machines; heating, ventilating,
and air conditioning systems; mammalian cell cultures; vegetables; human
nostrils and throats; and human and animal brain, skin, and lung
tissues. B. mandrillaris however, has not been isolated
from the environment but has been isolated from autopsy specimens of
infected humans and animals. Unlike N. fowleri, Acanthamoeba
and Balamuthia have only two stages, cysts
 and trophozoites
and trophozoites  , in their
life cycle. No flagellated stage exists as part of the life cycle.
The trophozoites replicate by mitosis (nuclear membrane does not remain
intact) , in their
life cycle. No flagellated stage exists as part of the life cycle.
The trophozoites replicate by mitosis (nuclear membrane does not remain
intact)  . The
trophozoites are the infective forms and are believed to gain entry into
the body through the lower respiratory tract, ulcerated or broken skin
and invade the central nervous system by hematogenous dissemination . The
trophozoites are the infective forms and are believed to gain entry into
the body through the lower respiratory tract, ulcerated or broken skin
and invade the central nervous system by hematogenous dissemination
 .
Acanthamoeba spp. and Balamuthia mandrillaris cysts and
trophozoites are found in tissue. .
Acanthamoeba spp. and Balamuthia mandrillaris cysts and
trophozoites are found in tissue.
CDC DPDx Parasite Image Library |
| |
A
B

Figure 26 Acanthamoeba sp. keratitis. A: Biopsy showing a cyst; B: cyst, at a larger magnification, with a characteristic shape, in corneal scraping.
CDC |
Acanthameba
Several species of free-living Acanthameba are pathogenic to man. They
normally reside in soil and can infect children who swallow dirt while
playing on the ground. In normal individuals, the infection may cause mild
disease (pharyngitis)
or remain asymptomatic, but in immunodeficient individuals, the organism may
penetrate the esophageal mucosa and reach the brain where it causes Granulomatous
Amebic Encephalitis (figure 26).
Granulomatous Amebic
Encephalitis
This is a rare infection that can affect the brain and disseminate to
the rest of the body. It can affect healthy people but is normally
associated with immunocompromized individuals (organ transplants,
lymphocyte disorders) and patients with diabetes, cancer, liver
cirrhosis, lupus and people who have used antibiotics and steroids
excessively.
Most cases are fatal. The
use of miltefosine is recommended by CDC.
Acanthameba Keratitis
Most cases of this disease in the United States occur in contact lens
users (1 to 33 cases per million). It results from improper storage and
cleaning of lenses in tap water.
|
Summary of
blood and tissue protozoa |
|
Organism |
Transmission |
Disease/symptoms |
Diagnosis |
Treatment |
|
Trypanosoma
brucei
|
Tsetse
fly. |
Sleeping
sickness; cardiac failure. |
Hemoflagellate
in blood or lymph node. |
Blood
stage: Suramin or petamidine isethionate; |
|
T.
cruzi |
Reduvid
(kissing) bug. |
Chagas
disease: megacolon, cardiac failure.
|
Hemoflagellate
in blood or tissue. |
CNS:
melarsoprol
Nifurtimox
and Benzonidazole. |
|
Leishmania
donovani |
Sand
fly |
Visceral leish-maniasis, granulo-matous skin lesions. |
Intracellular
(macrophages) leishmanial bodies.
|
Pentosam;
Pentamidine isethionate. |
|
L.
tropica |
Sand
fly. |
Cutaneous
lesions. |
As for
L. donovani. |
As for
L. donovani. |
|
L.
braziliensis |
Sand
fly |
Mucocutaneous
lesions. |
As for
L. donovani. |
As for
L. donovani. |
|
Plasmodium
falciparum
P. ovale, P. malariae and P. vivax |
Female
anopheline mosquito.
|
Malarial
paroxysm: chills, fever, headache, nausea cycles.
|
Plasmodia
in rbc, typical of the species involved. |
Quinine
derivatives
Proguanil
Lariam |
|
Babesia
microti
|
Tick |
Hemolytic
anemia, Jaundice and fever |
Typical
organism (Maltese cross) in rbc. |
None;
self resolving. |
|
Toxoplasma
gondii |
Oral
from cat fecal material;
or meat
|
Adult:
flu like;
congenital:
abortion, neonatal blindness and neuropathies.
|
Intracellular
(in macrophages) tachyzoites. |
Sulphonamides,
pyemethamine, possibly spiramycin (non-FDA).
|
|
Pneumocystis
jiroveci |
Cough
droplets |
Pneumonia
|
Pneumocystis
in sputum. |
Trimethoprim
and sulphamethoxazole. |
|
|

 |
 Return to the Parasitology Section of Microbiology and Immunology On-line
Return to the Parasitology Section of Microbiology and Immunology On-line
This page last changed on
Tuesday, February 24, 2015
Page maintained by
Richard Hunt
|


 Figure 21D
Figure 21D
 Figure 24A
Figure 24A  Figure 24D
Figure 24D
 Figure 25 A
Figure 25 A  Figure 25C
Figure 25C  Figure
25E
Figure
25E 











