|
x |
x |
|
 |
 |
|
INFECTIOUS
DISEASE |
BACTERIOLOGY |
IMMUNOLOGY |
MYCOLOGY |
PARASITOLOGY |
VIROLOGY |
|
|
BACTERIOLOGY - CHAPTER TWENTY ONE
RICKETTSIA,
ORIENTIA, EHRLICHIA, ANAPLASMA, COXIELLA AND BARTONELLA
Dr. Gene Mayer
Professor Emeritus
University of South Carolina School of Medicine
|
|
SLOVAK |
|
TURKISH |
|
|
|
|
Let us know what you think
FEEDBACK |
|
SEARCH |
 |
|
Most images on this page come
from the Centers for Disease Control |
|
TEACHING OBJECTIVES
To describe the interactions
of the Rickettsia, Ehrlichia, Coxiella and Bartonella with the host
cell
To describe the pathogenesis,
epidemiology and clinical syndromes associated with Rickettsia, Ehrlichia,
Coxiella and Bartonella
To discuss the methods for
treatment, prevention and control of rickettsial diseases
KEY WORDS
Reservoir
Vector
Rocky Mountain spotted fever
Ehrlichiosis
Rickettsialpox
Scrub typhus
Epidemic typhus
Murine typhus
Q fever
Trench fever
Cat-scratch disease
Transovarian passage
Weil-Felix test
Brill-Zinsser disease
Morula |
Rickettsial infections have played
a significant role in the history of Western civilization. Epidemic typhus has
been known since the 16th century and it has long been associated
with famine and war. The outcome of several wars was influenced by epidemic
typhus. Typhus killed or caused great suffering to over 100,000 people in the
two World Wars. In spite of its long history, it was not until the early part of
the 20th century that the causative agent was determined. Howard
Ricketts described the causative agent of Rocky Mountain Spotted Fever and was
able to culture it in laboratory animals. Others then realized that the
causative agent of epidemic typhus was related to the organism that Ricketts
described. After the discovery of the importance of arthropod vectors in the
spread of typhus, vector control measures were instituted to control the
disease. However, as Hans Zinsser has pointed out, typhus is not dead.
The Rickettsia, Ehrlichia,
Anaplasma and
Coxiella are all small obligate intracellular parasites which were once
thought to be part of the same family. Now, however, they are considered to be
distinct unrelated bacteria. Like the Chlamydia these bacteria were once
thought to be viruses because of their small size and intracellular life cycle.
However, they are true bacteria, structurally similar to Gram negative bacteria.
They are small Gram negative coccobacilli that are normally stained with Giemsa since
they stain poorly with the Gram stain. Although these bacteria are able to make
all the metabolites necessary for growth, they have an ATP transport system that
allows them to use host ATP. Thus, they are energy parasites as long as ATP is
available from the host.
All of these organisms are
maintained in animal and arthropod reservoirs and, with the exception of Coxiella,
are transmitted by arthropod vectors ( e.g., ticks, mites, lice or
fleas). Humans are accidentally infected with these organisms. The reservoirs,
vectors and major diseases caused by these organisms are summarized in Table 1
(Adapted from: Murray,et al. Medical Microbiology).
|
Table 1 |
|
Disease |
Organism |
Vector |
Reservoir |
|
Rocky Mountain spotted fever |
R. rickettsii |
Tick |
Ticks, wild rodents |
|
Ehrlichiosis |
E. chaffeensis
E. ewingii
|
Tick |
Deer
Small mammals |
|
Anaplasmosis |
A. phagocytophilum |
Tick |
Deer
Small mammals |
|
Rickettsial pox |
R. akari |
Mite |
Mites, wild rodents |
|
Scrub typhus |
R. tsutsugamushi |
Mite |
Mites, wild rodents |
|
Epidemic typhus |
R. prowazekii |
Louse |
Humans, squirrel fleas, flying
squirrels |
|
Murine typhus |
R. typhi |
Flea |
Wild rodents |
|
Q fever |
C. burnetii |
None |
Cattle, sheep, goats, cats |
|
|
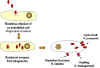 Figure 1
Figure 1
Rickettsial and Orientia infection of endothelial cells |
Rickettsia
and Orientia
Orientia were formerly
called Rickettsia
Replication
The Rickettsia
preferentially infect endothelial cells lining the small blood vessels by
parasite-induced phagocytosis (figure 1). Once in the host cell, the bacteria lyse the
phagosome membrane with a phospholipase and get into the cytoplasm where they
replicate. The mode of exit from the host cell varies depending upon the species. R.
prowazekii exits by cell lysis while R. rickettsii get extruded
from the cell through local projections (filopodia). F actin in the host cell
associates with R. rickettsii and the actin helps to "push" the
bacteria through the filopdia (figure 2). O. tsutsugamushi exits by budding
through the cell membrane and remains enveloped in the host cell membrane as
it infects other cells.
Antigenic structure
Based on their antigenic composition, the Rickettsia are divided into several
groups. The organisms in each group, the diseases caused by the organisms and
their geological distribution are summarized in Table 2 (Adapted from: Murray, et
al., Medical Microbiology).
|
Table 2 |
|
Spotted fever group |
|
Organism |
Disease |
Distribution |
|
R. rickettsii |
Rocky Mountain spotted fever |
Western hemisphere |
|
R. akari |
Rickettsial pox |
USA, former Soviet Union |
|
R. conorii |
Boutonneuse fever |
Mediterranean countries, Africa,
India, Southwest Asia |
|
R. sibirica |
Siberian tick typhus |
Siberia, Mongolia, northern China |
|
R. australis |
Australian tick typhus |
Australia |
|
R. japonica |
Oriental spotted fever |
Japan |
|
Typhus group |
|
Organism |
Disease |
Distribution |
|
R. prowazekii |
Epidemic typhus
Recrudescent typhus
Sporadic typhus |
South America and Africa
Worldwide
United States |
|
R. typhi |
Murine typhus |
Worldwide |
|
Scrub typhus group |
|
Organism |
Disease |
Distribution |
|
O. tsutsugamushi |
Scrub typhus |
Asia, northern Australia, Pacific
Islands |
Pathogenesis and Immunity
Pathogenesis is primarily due to destruction of the
infected cells by the replicating
bacteria. Destruction of endothelial cells results in leakage of blood and
subsequent organ and tissue damage due to loss of blood into the tissue
spaces. No evidence for immunopathological damage has been obtained. Both
humoral and cell mediated immunity are important in recovery from infection.
Antibody-opsonized Rickettsia are phagocytosed and killed by macrophages and
delayed type hypersensitivity develops following rickettsial infections.
|
Figure 2
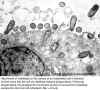 Attachment of rickettsiae to the surface of an endothelial cell is followed by their entry into the cell via
rickettsia- induced phagocytosis. Following phagocytosis, the phagosome membrane (arrow) is lost and the rickettsiae escape into the host cell cytoplasm.
Attachment of rickettsiae to the surface of an endothelial cell is followed by their entry into the cell via
rickettsia- induced phagocytosis. Following phagocytosis, the phagosome membrane (arrow) is lost and the rickettsiae escape into the host cell cytoplasm.
© Vsevolod Popov and David H. Walker, University of Texas Medical Branch at Galveston, Galveston, Texas
USA and
The MicrobeLibrary |
 Following release from the phagosomes, rickettsiae grow free in the cytoplasm of cultured cells, dividing by binary fission (seen at arrows). The inset photo highlights the outer and inner membranes of
rickettsia. © Vsevolod Popov and David H. Walker, University of Texas Medical Branch at Galveston, Galveston, Texas USA
and
The MicrobeLibrary
Following release from the phagosomes, rickettsiae grow free in the cytoplasm of cultured cells, dividing by binary fission (seen at arrows). The inset photo highlights the outer and inner membranes of
rickettsia. © Vsevolod Popov and David H. Walker, University of Texas Medical Branch at Galveston, Galveston, Texas USA
and
The MicrobeLibrary
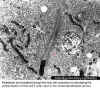 Rickettsiae are propelled through the host cell cytoplasm by stimulating the
polymerization of host cell F-actin, seen in the comet-like 'tail' (arrow).
© Vsevolod Popov and David H. Walker, University of Texas Medical Branch at Galveston, Galveston, Texas USA
and
The MicrobeLibrary
Rickettsiae are propelled through the host cell cytoplasm by stimulating the
polymerization of host cell F-actin, seen in the comet-like 'tail' (arrow).
© Vsevolod Popov and David H. Walker, University of Texas Medical Branch at Galveston, Galveston, Texas USA
and
The MicrobeLibrary
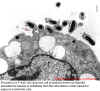 Propulsion by F-actin into long host cell projections known as filopodia precedes the release of rickettsiae from the cell surface or their spread to adjacent endothelial cells.
© Vsevolod Popov and David H. Walker, University of Texas Medical Branch at Galveston, Galveston, Texas USA
and
The MicrobeLibrary
Propulsion by F-actin into long host cell projections known as filopodia precedes the release of rickettsiae from the cell surface or their spread to adjacent endothelial cells.
© Vsevolod Popov and David H. Walker, University of Texas Medical Branch at Galveston, Galveston, Texas USA
and
The MicrobeLibrary
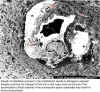 Growth of rickettsiae (arrows) in the endothelium results in damage to vascular integrity and thus the leakage of fluid into a vital organ such as the brain. The accumulation of fluid (edema) in the perivascular space (asterisks) may result in clinical encephalitis.
© Vsevolod Popov and David H. Walker, University of Texas Medical Branch at Galveston, Galveston, Texas USA
and
The MicrobeLibrary
Growth of rickettsiae (arrows) in the endothelium results in damage to vascular integrity and thus the leakage of fluid into a vital organ such as the brain. The accumulation of fluid (edema) in the perivascular space (asterisks) may result in clinical encephalitis.
© Vsevolod Popov and David H. Walker, University of Texas Medical Branch at Galveston, Galveston, Texas USA
and
The MicrobeLibrary
 Gamma interferon and tumor necrosis factor alpha, substances secreted by host immune cells, "activate" the infected endothelial cell to kill intracellular rickettsiae via the creation of autophagosomes. Later, fusion of lysosomes with
autophagosomes results in the digestion of dying rickettsia
(arrow).
© Vsevolod Popov and David H. Walker, University of Texas Medical Branch at Galveston, Galveston, Texas USA
and
The MicrobeLibrary
Gamma interferon and tumor necrosis factor alpha, substances secreted by host immune cells, "activate" the infected endothelial cell to kill intracellular rickettsiae via the creation of autophagosomes. Later, fusion of lysosomes with
autophagosomes results in the digestion of dying rickettsia
(arrow).
© Vsevolod Popov and David H. Walker, University of Texas Medical Branch at Galveston, Galveston, Texas USA
and
The MicrobeLibrary
|
| |
|
|
Rickettsia rickettsii (Rocky
Mountain Spotted Fever)
Epidemiology
Rocky
Mountain Spotted Fever is the most common rickettsial disease in the United
States with 400 - 700 cases occurring annually.
While the disease was originally described in the Rocky Mountain states, it
is now most common in the South Central states, including South Carolina
(figure 4).
The organism is
transmitted by the bite of an infected tick with most infections occurring
from April through September because of more frequent human contact with
ticks at this time of the year. The Rickettsia in the tick are in a dormant state
and must be activated by the warm blood meal. They are then released into the saliva of
the tick. Thus, prolonged exposure (24 - 48 hrs) to an infected tick must
occur before the organisms can infect the human host. The principal
reservoir for R. rickettsii is the ixodid (hard) tick in which
transovarian passage occurs. Wild rodents can become infected and act as a
reservoir for the bacteria but they are not considered to be the main
reservoir.
|
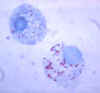 Figure 3
Figure 3
Gimenez stain of tick hemolymph cells infected with R. rickettsii
CDC |
|
Figure 4
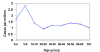 Average annual incidence of Rocky Mountain spotted fever by age
group, 1993-1996 CDC
Average annual incidence of Rocky Mountain spotted fever by age
group, 1993-1996 CDC |
 Reported cases of Rocky Mountain spotted fever in the United States, 1942-1996
CDC
Reported cases of Rocky Mountain spotted fever in the United States, 1942-1996
CDC
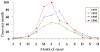 Seasonal distribution of reported cases of Rocky Mountain spotted fever, 1993-1996
CDC
Seasonal distribution of reported cases of Rocky Mountain spotted fever, 1993-1996
CDC
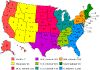 Number of reported cases of Rocky Mountain spotted fever by state and
region, 1994-1998 CDC
Number of reported cases of Rocky Mountain spotted fever by state and
region, 1994-1998 CDC
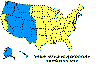 Approximate distribution of the American dog tick CDC
Approximate distribution of the American dog tick CDC

Rocky Mountain wood tick (Dermacentor
andersoni) CDC
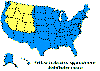 Approximate distribution of the Rocky Mountain
wood tick CDC
Approximate distribution of the Rocky Mountain
wood tick CDC
|
 American dog tick (Dermacentor variabilis) CDC
American dog tick (Dermacentor variabilis) CDC |
 Figure
5 Figure
5
Generalized Life Cycle of Dermacentor variabilis and Dermacentor andersoni Ticks (Family
Ixodidae) CDC |
Clinical syndromes
Rocky
Mountain spotted fever begins with the abrupt onset of fever, chills
headache and
myalgia usually 2 - 12 days after the tick bite. Patients may not
recall being bitten by a tick. Rash usually (90% of cases) appears 2 - 3 days
later. The rash begins on the hands and feet and spreads centripetally
towards the trunk. Rash on the palms and soles is common. Initially, the rash
is maculopapular but in the
later stages may become
petechial and
hemorrhagic (figure 6).
Complications
from widespread
vasculitis can include gastrointestinal symptoms,
respiratory failure, seizures, coma and acute renal failure. Complications
occur most frequently in cases in which the rash does not develop, since
treatment is usually delayed. Mortality rate in untreated patients is 20%.
|
| Figure 6 |
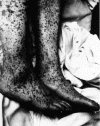 Characteristic spotted rash of late-stage Rocky Mountain spotted fever on legs of a patient, ca. 1946
CDC
Characteristic spotted rash of late-stage Rocky Mountain spotted fever on legs of a patient, ca. 1946
CDC
 Early (macular) rash on sole of foot
CDC
Early (macular) rash on sole of foot
CDC
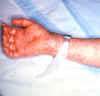 Late (petechial) rash on palm and forearm
CDC
Late (petechial) rash on palm and forearm
CDC
|
| |
Laboratory diagnosis
Initial diagnosis should be made on clinical grounds and treatment should
not be delayed until laboratory confirmation is obtained. A fluorescent
antibody test to detect antigen in skin punch biopsies is the fastest way to
confirm a diagnosis. However, this test is available only in reference
laboratories. PCR based methods are also available but limited to reference
laboratories. The Weil-Felix test, which is an agglutination test to detect
antibodies that cross react with Proteus vulgaris, is no longer
recommended. The primary laboratory diagnostic tool is serology. Indirect
fluorescent antibody tests (figure 7) and latex agglutination tests are available for
serological diagnosis of Rocky Mountain spotted fever.
|
 Figure 7
Figure 7
IFA reaction of a positive human serum on Rickettsia rickettsii grown in chicken yolk sacs, 400X
CDC
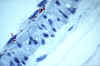 Figure 8
Figure 8
Red structures indicate immunohistological staining of
Rickettsia rickettsii in endothelial cells of a blood vessel from a patient with fatal RMSF
CDC |
Treatment, prevention and
control
R. rickettsii is susceptible to tetracyclines and
chloramphenicol. Prompt treatment is necessary since morbidity and mortality
increases if treatment is delayed. No vaccine is available. Prevention of
tick bites (protective clothing, insect repellents, etc.) and prompt removal
of ticks are the best preventative measures. It is not feasible to attempt
to control the tick reservoir.
Rickettsia akari (rickettsialpox)
Epidemiology
R. akari
is found in the United States and sporadic infections occur. The vector is a
mouse mite and the reservoirs are mites and mice. In mites, the bacteria are
maintained by transovarian transmission. Humans are accidentally infected.
Clinical syndromes
Rickettsialpox is typically a mild disease that has two phases. In the first
phase a papule develops at the site of the mite bite and quickly ulcerates
and forms an
eschar. This initial phase occurs approximately 1 week after
the bite. After an incubation time of 7 to 24 days, the second phase of the
disease occurs. This phase is characterized by sudden onset of fever, chills
headache and myalgia and is followed 2 to 3 days later with a generalized
rash. The rash is papulovesicular and crusts over in the later stages. The
pox heal within 2 to 3 weeks without scarring. Fatalities are rare.
Laboratory diagnosis
Not
available except in certain reference laboratories
Treatment and prevention and
control
Tetracycline and chloramphenicol can speed up recovery. Measures
aimed at controlling mouse populations help to prevent the disease.
|
| |
Rickettsia prowazekii
(Epidemic typhus or louse-borne typhus)
Epidemiology
Epidemic
typhus is a disease transmitted by the human body louse. When an infected
louse bites a human, it defecates and the bacteria are found in the feces.
Irritation caused by the bite causes the person to scratch the bite and
thereby to inoculate the bacteria into abraded skin. Unlike the other rickettsial diseases, humans are the primary reservoir for R. prowazekii.
Epidemic typhus occurs among people living in crowded, unsanitary
conditions such as those found in wars, famine and natural disasters. Transovarian transmission in the louse does not occur since lice die several
weeks after being infected. The disease occurs sporadically in the United
States, primarily in the Eastern states where the reservoirs are flying
squirrels and their fleas. The fleas are the vector that transmit the
disease.
Clinical syndromes
a. Epidemic typhus is
characterized by sudden onset of fever, chills, headache
myalgia
and
arthralgia, after an average incubation period of 8 days. Approximately 7
days later, a rash develops in most patients. The rash is
maculopapular but
can be
petechial or hemorrhagic. In contrast to the rash seen with Rocky
Mountain Spotted Fever, the rash in epidemic typhus develops on the trunk
first and spreads to the extremities (centrifugal spread). Complications
include: myocarditis, stupor and delirium. The name typhus comes from the
Greek for "smoke" underscoring the fact that stupor and delirium often
complicate the disease. Recovery may take several months. The mortality
rate varies but can be quite high (60 - 70%) in some epidemics.
b. Brill-Zinsser disease
is recrudescent epidemic typhus. It occurs decades after the initial
infection. In the United States it is most commonly seen in those who were
exposed to epidemic typhus in World War II. The clinical course of the
disease is similar to epidemic typhus but is milder and recovery is
faster. The skin rash is rarely seen. Diagnosis is made on the basis of a
fever with unknown origin and a history of previous exposure to epidemic
typhus.
Laboratory diagnosis
Diagnosis should be made on clinical findings and treatment should begin
before laboratory confirmation.
Weil-Felix antibodies are produced but the
test is not recommended. Serology is the primary laboratory test used for
diagnosis of R. prowazekii. Indirect fluorescent antibody tests and
latex agglutination tests are available. Patients with epidemic typhus
initially have an IgM response followed by IgG antibodies whereas patients
with Brill-Zinsser disease initially have an
anamnestic IgG response.
Isolation of the organism is possible but dangerous.
Treatment, prevention and
control
Tetracyclines and chloramphenicol are highly effective. Louse
control measures can prevent infection. A killed typhus vaccine is available
and is recommended for use in high-risk populations.
|
| |
Rickettsia typhi (Murine or
endemic typhus)
Epidemiology
Murine typhus
occurs worldwide with approximately 40 - 60 cases being reported in the United
States annually. Rats are the primary reservoir for the disease which is
transmitted by the rat flea vector. The normal cycle is rat to flea to rat
and humans are accidentally infected. Since there is no transovarian
transfer in the flea, the flea is not a reservoir for the disease. The cat
flea can also be a vector for the disease in the United States. The bacteria
are in the flea feces and are inoculated into abraded skin by scratching the
area irritated by the bite.
Clinical syndromes
The
symptoms of fever, chills headache and myalgia appear abruptly 1 - 2 weeks
after infection. A rash develops in many but not all cases. The rash begins
on the trunk and spreads to the extremities, unlike the rash seen in Rocky
Mountain Spotted Fever. The disease is mild and resolves within 3 weeks even
if untreated.
Laboratory diagnosis
A
serological indirect fluorescent antibody test is used to detect antibodies
to R. typhi.
Treatment, prevention and
control
Tetracyclines and chloramphenicol are effective. Controlling the
rodent reservoir is useful in preventing infection. A vaccine is not
available.
|
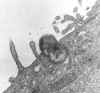 Figure
9 Figure
9
Phagocytosis of Rickettsia tsutsugamushi by mouse peritoneal mesothelial
cell. CDC/Dr. Edwin P. Ewing, Jr. epe1@cdc.gov |
Orientia (Rickettsia) tsutsugamushi
(Scrub typhus)
Epidemiology
Scrub typhus
occurs in Asia, Australia and the Pacific Islands. The disease is
transmitted to humans by chiggers, the larval form of a mite. The mite
is both the reservoir and the vector and passes the bacteria transovarially.
Rodents can also act as a reservoir. The normal cycle is mite to rodent to
mite; humans are accidentally infected.
Clinical syndromes
The
disease is characterized by sudden onset of fever, chills headache and myalgia 1 - 3 weeks after contracting the bacteria. A maculopapular rash
develops 2 - 3 days later. The rash appears first on the trunk and spreads
to the extremities (centrifugal spread). Mortality rate in outbreaks are
variable.
Laboratory diagnosis
Serological tests for antibody are available.
Treatment, prevention and
control
Tetracyclines and chloramphenicol are effective. Avoiding exposure
to chiggers will prevent the disease.
|
|
|
Ehrlichia
and Anaplasma
Replication
The Ehrlichia
and Anaplasma preferentially infect leukocytes. They enter the cell by phagocytosis and once
in the host cell they inhibit phagolysosome fusion. The organisms grow within
the membrane-bound phagosome and are released by lysis of the cell (figure 10). The
inclusion body containing the organisms is called a morula.
Epidemiology
The Ehrlichia
are divided into three groups based on genetic homology. Table 3 (Adapted
from:
Murray, et al., Medical Microbiology)
summarizes the human diseases caused by the Ehrlichia and Analplasma, the vectors,
reservoirs and the geographic distributions.
|
 Figure
10 Figure
10
Infection of leukocytes by Ehrlichia |
Figure 11
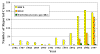 Reported Cases of Ehrlichiosis in the United States
CDC
Reported Cases of Ehrlichiosis in the United States
CDC |
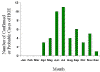 Approximate seasonal distribution of HGE in the United States
CDC
Approximate seasonal distribution of HGE in the United States
CDC
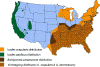 Areas where human ehrlichiosis may occur based on approximate distribution of
vector tick species CDC
Areas where human ehrlichiosis may occur based on approximate distribution of
vector tick species CDC
|
| |
|
Table 3 |
|
Organism |
Disease |
Vector |
Reservoir |
Distribution |
|
E chaffeensis |
Human monocytic ehrlichiosis |
Lone Star tick |
White tailed deer |
Southeastern, Mid-Atlantic and
South Central United States |
|
E. ewingii |
Primarily a dog disease
Human granulocytic ehrlichiosis |
Lone Star tick |
White tailed deer |
Southeastern, Mid-Atlantic and
South Central United States |
|
A. phagocytophilum |
Human granulocytic anaplasmosis (human
anaplasmosis) |
Deer and dog ticks |
Small mammals |
Wisconsin, Minnesota,
Connecticut |
|
E. sennetsu |
Sennetsu fever |
Unknown |
Unknown |
Japan |
|
| |
Ehrlichia chaffeensis
(human monocytic ehrlichiosis)
Clinical syndromes
The
disease resembles Rocky Mountain Spotted Fever, except that the rash does not
develop in most (80%) patients. In addition, leukopenia is observed due to
destruction of the leukocytes. Mortality is low (5%).
Laboratory diagnosis
Microscopic observation of morula in blood smears is rare and
,although
culture is possible, it is rarely attempted. Serological test are available
and are the most commonly employed test. DNA probes are available and may
replace serological tests.
Treatment, prevention and
control
Patients should be treated with doxycycline. Avoidance of tick
infected areas and protective measures (clothing and insect repellents) can
prevent the disease.
|
Figure 12
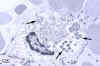 Electron-photomicrograph of morulae in a bone marrow leukocyte in a patient
with ehrlichiosis. Arrows indicate individual ehrlichiae
CDC
Electron-photomicrograph of morulae in a bone marrow leukocyte in a patient
with ehrlichiosis. Arrows indicate individual ehrlichiae
CDC |
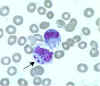 Ehrlichia chaffeensis primarily infects
mononuclear leukocytes (predominantly monocytes and
macrophages), but may also be seen occasionally in the granulocytes of some patients with severe disease.
Ehrlichia chaffeensis primarily infects
mononuclear leukocytes (predominantly monocytes and
macrophages), but may also be seen occasionally in the granulocytes of some patients with severe disease.
(Morulae in cytoplasm of monocyte)
CDC
 Lone star tick (Amblyomma americanum) CDC
Lone star tick (Amblyomma americanum) CDC
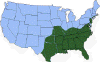 Approximate distribution of the lone star tick
CDC
Approximate distribution of the lone star tick
CDC
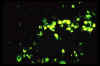 IFA of Ehrlichia chaffeensis in DH82 cells, 400X CDC
IFA of Ehrlichia chaffeensis in DH82 cells, 400X CDC
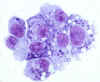 Diff-Quik Stain of Ehrlichia chaffeensis in DH82 cells, 1000X
CDC
Diff-Quik Stain of Ehrlichia chaffeensis in DH82 cells, 1000X
CDC
 Ehrlichia sp. develop within host cell vacuoles first as reticulate cells (RC) and then as dense-core cells (DC). A vacuole containing an ehrlichial microcolony
is called a morula. Several morulae are seen in this host cell, including one filled with what appear to be dead ehrlichiae (shown at the arrow).
Ehrlichia sp. develop within host cell vacuoles first as reticulate cells (RC) and then as dense-core cells (DC). A vacuole containing an ehrlichial microcolony
is called a morula. Several morulae are seen in this host cell, including one filled with what appear to be dead ehrlichiae (shown at the arrow).
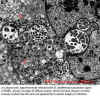 A cultured cell, experimentally infected with E. chaffeensis (causative agent of HME), shows morulae of different sizes. Small morulae (shown at white arrows) contain few RC and are apparently in earlier stages of infection.
A cultured cell, experimentally infected with E. chaffeensis (causative agent of HME), shows morulae of different sizes. Small morulae (shown at white arrows) contain few RC and are apparently in earlier stages of infection.
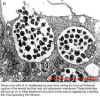 Dense-core cells of E. chaffeensis are seen exiting the host cell following rupture of the morula and the host cell cytoplasmic membrane. These ehrlichiae will now go on to infect additional host cells or they may be ingested by a feeding tick, thus spreading the infection.
Dense-core cells of E. chaffeensis are seen exiting the host cell following rupture of the morula and the host cell cytoplasmic membrane. These ehrlichiae will now go on to infect additional host cells or they may be ingested by a feeding tick, thus spreading the infection.
|
| |
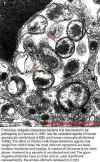 A number of the bacteria are clustered in a vacuole in an infected host cell. The gram negative
ehrlichiae have an inner and an outer membrane represented by the arrows. (All bars represent 0.5 mm.)
A number of the bacteria are clustered in a vacuole in an infected host cell. The gram negative
ehrlichiae have an inner and an outer membrane represented by the arrows. (All bars represent 0.5 mm.) |
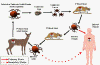 Figure 13
Figure 13
Proposed life cycle for the agent of Human Granulocytic Ehrlichiosis
CDC |
Ehrlichia ewingii and Anaplasma phagocytophilum (human
granulocytic ehrlichiosis)
Clinical syndromes
The
disease is similar to human monocytic ehrlichiosis except that mortality
rates may be higher (10%)
Laboratory diagnosis
Same
as E. chaffeensis
Treatment, prevention and
control
Same as E. chaffeensis
|
Figure 14
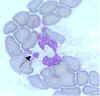 The pathogen that causes human granulocytic ehrlichiosis
(HGE) primarily infects granulocytes (neutrophils and rarely
eosinophils). The pathogen is often referred to as the agent of HGE or the HGE agent. This species is very similar, or likely identical, to E.
phagocytophila and E. equi. (Morulae in cytoplasm of neutrophil) CDC
The pathogen that causes human granulocytic ehrlichiosis
(HGE) primarily infects granulocytes (neutrophils and rarely
eosinophils). The pathogen is often referred to as the agent of HGE or the HGE agent. This species is very similar, or likely identical, to E.
phagocytophila and E. equi. (Morulae in cytoplasm of neutrophil) CDC |
 Blacklegged tick (Ixodes scapularis) CDC
Blacklegged tick (Ixodes scapularis) CDC
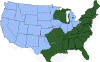 Approximate distribution of the blacklegged tick CDC
Approximate distribution of the blacklegged tick CDC
 Western blacklegged tick (Ixodes pacificus) CDC
Western blacklegged tick (Ixodes pacificus) CDC
|
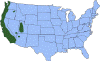 Figure
15
Approximate distribution of the western blacklegged tick
CDC Figure
15
Approximate distribution of the western blacklegged tick
CDC |
Ehrlichia sennetsu (Sennetsu
fever)
Clinical syndromes
The
disease resembles infectious mononucleosis with fever, lethargy, cervical lymphadenopathy, increased number of peripheral blood mononuclear cells and
atypical lymphocytes.
Laboratory diagnosis
Serological tests are available
Treatment
Tetracycline has
been used but the disease is benign with no fatalities or serious
complications.
|
|
WEB RESOURCES
CDC
Q fever site |
COXIELLA
Coxiella burnetii (Q
fever)
[Q for query]
Replication
C. burnetii infects
macrophages (figure 16) and survives in the phagolysosome where they multiply. The
bacteria are released by lysis of the cells and phagolysosomes.
Pathogenesis and immunity
Infection occurs by inhalation of airborne particles. The organism
multiplies in the lungs and is disseminated to other organs. Pneumonia and
granulomatous hepatitis are observed in patients with severe infections. In
chronic disease, immune complexes may play a role in pathogenesis. Phase
variation occurs in the LPS of C. burnetii. In acute disease, antibodies
are produced against the phase II antigen. In chronically infected patients,
antibodies to both phase I and phase II antigens are observed. Cellular
immunity is important in recovery from the disease.
Epidemiology
C.
burnetii is extremely stable in the environment and has
"spore-like"
characteristics. C. burnetii infects a wide range of animals including
goats sheep cattle and cats. The organism is found in the placenta and in the
feces of infected livestock. The organisms persist in contaminated soil which is
a focus for infection. C. burnetii is also passed in milk and people
who consume non-pasteurized milk can become infected. Arthropods are not
common vectors for transmission of C. burnetii in humans but ticks
are a primary vector for transmission among veterinary species.
C. burnetii is found worldwide and
infection is common in ranchers, veterinarians, abattoir workers and others
associated with cattle and livestock.
Clinical syndromes
The disease can be mild and asymptomatic and is often undiagnosed. The disease
can be acute or chronic. In acute Q fever, the patient presents with headache
fever, chills and myalgia. Respiratory symptoms are usually mild
("atypical pneumonia"). Hepatomegaly and splenomegaly may be observed. Granulomas can be
seen in histological sections of most patients with Q fever. Chronic Q fever
typically presents as endocarditis generally on a damaged heart valve.
Prognosis of chronic Q fever is not good.
Laboratory diagnosis
Serology is most commonly used to diagnose Q fever. Antibodies to phase II
antigen is used to diagnose acute disease and antibodies to both phase I and
phase II antigens to diagnose chronic disease.
Treatment, prevention and
control
Tetracycline in used to treat acute Q fever. Chronic disease is
treated by a combination of antibiotics. A vaccine is
available in some countries, such as Australia, but it has not been approved
for use in the United States.
|
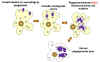 Figure 16
Figure 16
Infection of Macrophages by Coxiella |
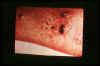 Figure 17
Figure 17
Bartonellosis- a bacterial onfection caused by Bartonella
bacilliformis and found in South America. The infection can occur as
either an acute febrile anemia (Oroya fever) or as a chronic cutaneous
eruption (Verruga peruana). © Dr
V. Lloyd, Mount Allison University
|
Bartonella
Microbiology
The Bartonella
are small, Gram-negative aerobic bacilli that are difficult to grow in
culture. They are found in many different animals but they cause no apparent
disease in animals. Insects are thought to be vectors in human disease. Some
species are able to infect erythrocytes while others simply attach to host
cells. Table 4 (Adapted from: Murray, et al., Medical Microbiology) summarizes the organisms and the diseases they cause.
|
Table 4 |
|
Organism |
Disease |
|
B. quintana (formerly Rochalimaea
quintana) |
Trench fever (shin-bone fever, 5
day fever), bacillary angiomatosis, bacillary peliosis endocarditis |
|
B. henselae |
Cat-scratch disease, bacillary
angiomatosis, bacillary peliosis endocarditis |
|
B. bacilliformis |
Oroya fever (bartonellosis,
Carrion's disease) |
|
B. elizabethae |
Endocarditis (rare) |
Bartonella quintana (Trench
fever)
Epidemiology
Trench fever
is a disease associated with war. The vector is the human body louse and
there is no known reservoir except man. Transovarian transmission in the
louse does not occur. The organism is found in the feces of the louse and is
inoculated into humans by scratching. The cycle is human to louse to human.
Clinical syndromes
Infection with B. quintana can result in asymptomatic to severe
debilitating illness. Symptoms include fever, chills, headache and severe
pain in the tibia. A maculopapular rash may or may not appear on the trunk.
The symptoms may reappear at five day intervals and thus the disease is also
called five day fever. Mortality rates are very low.
Laboratory diagnosis
Serological tests are available but only in reference laboratories. PCR
based tests have been developed.
Treatment, prevention and
control
Various antibiotics have been used to treat trench fever. Measures
to control the body louse are the best form of prevention.
|
| |
Bartonella henselae -
(Cat-scratch disease)
Epidemiology
Cat-scratch disease is acquired after exposure to cats (scratches, bites,
and possible cat fleas).
Clinical syndromes
The disease in usually benign, characterized by chronic regional lymphadenopathy.
Laboratory diagnosis
Serological tests are available
Treatment
Cat-scratch disease does not appear to respond to antimicrobial therapy.
|
|
|
 Return to the Bacteriology Section of the
Microbiology and Immunology On-line Textbook
Return to the Bacteriology Section of the
Microbiology and Immunology On-line Textbook
This page last changed on
Sunday, March 06, 2016
Page maintained by
Richard Hunt
|

 Attachment of rickettsiae to the surface of an endothelial cell is followed by their entry into the cell via
rickettsia- induced phagocytosis. Following phagocytosis, the phagosome membrane (arrow) is lost and the rickettsiae escape into the host cell cytoplasm.
Attachment of rickettsiae to the surface of an endothelial cell is followed by their entry into the cell via
rickettsia- induced phagocytosis. Following phagocytosis, the phagosome membrane (arrow) is lost and the rickettsiae escape into the host cell cytoplasm. Figure 3
Figure 3 American dog tick (Dermacentor variabilis) CDC
American dog tick (Dermacentor variabilis) CDC Figure
5
Figure
5 Figure 7
Figure 7 Figure
9
Figure
9 Figure
10
Figure
10 Reported Cases of Ehrlichiosis in the United States
CDC
Reported Cases of Ehrlichiosis in the United States
CDC Electron-photomicrograph of morulae in a bone marrow leukocyte in a patient
with ehrlichiosis. Arrows indicate individual ehrlichiae
CDC
Electron-photomicrograph of morulae in a bone marrow leukocyte in a patient
with ehrlichiosis. Arrows indicate individual ehrlichiae
CDC A number of the bacteria are clustered in a vacuole in an infected host cell. The gram negative
ehrlichiae have an inner and an outer membrane represented by the arrows. (All bars represent 0.5 mm.)
A number of the bacteria are clustered in a vacuole in an infected host cell. The gram negative
ehrlichiae have an inner and an outer membrane represented by the arrows. (All bars represent 0.5 mm.) Figure 13
Figure 13 The pathogen that causes human granulocytic ehrlichiosis
(HGE) primarily infects granulocytes (neutrophils and rarely
eosinophils). The pathogen is often referred to as the agent of HGE or the HGE agent. This species is very similar, or likely identical, to E.
phagocytophila and E. equi. (Morulae in cytoplasm of neutrophil) CDC
The pathogen that causes human granulocytic ehrlichiosis
(HGE) primarily infects granulocytes (neutrophils and rarely
eosinophils). The pathogen is often referred to as the agent of HGE or the HGE agent. This species is very similar, or likely identical, to E.
phagocytophila and E. equi. (Morulae in cytoplasm of neutrophil) CDC Figure
15
Approximate distribution of the western blacklegged tick
CDC
Figure
15
Approximate distribution of the western blacklegged tick
CDC Figure 16
Figure 16 Figure 17
Figure 17




























