|
x |
x |
|
 |
 |
|
INFECTIOUS
DISEASE |
BACTERIOLOGY |
IMMUNOLOGY |
MYCOLOGY |
PARASITOLOGY |
VIROLOGY |
|
TURKISH |
BACTERIOLOGY - CHAPTER SIX
ANTIBIOTICS - PROTEIN SYNTHESIS, NUCLEIC ACID SYNTHESIS AND
METABOLISM
Dr Gene Mayer
Emeritus Professor
University of South Carolina School of Medicine
|
|
EN
ESPANOL-SPANISH |
|
SHQIP-ALBANIAN |
|
FARSI |
|
|
|
Let us know what you think
FEEDBACK |
|
SEARCH |
|
|
|

 |
|
Logo image © Jeffrey
Nelson, Rush University, Chicago, Illinois and
The MicrobeLibrary |
|
TEACHING OBJECTIVES
To describe the mode of action of antibacterial
chemotherapeutic agents
To discuss antibiotic susceptibility testing
To review the mechanisms by which bacteria express
resistance to antibiotics |
Major Principles and Definitions
Selectivity
Clinically effective antimicrobial agents
all exhibit selective toxicity toward the bacterium rather than the host. It is
this characteristic that distinguishes antibiotics from disinfectants. The basis
for selectivity will vary depending on the particular antibiotic. When
selectivity is high the antibiotics are normally not toxic. However, even highly
selective antibiotics can have side effects.
Therapeutic Index
The therapeutic index is defined as
the ratio of the dose toxic to the host to the effective therapeutic dose. The
higher the therapeutic index the better the antibiotic.
Categories of Antibiotics
Antibiotics are categorized as bactericidal if they kill the susceptible bacteria or bacteriostatic
if they reversibly inhibit the growth of bacteria. In general the use of
bactericidal antibiotics is preferred but many factors may dictate the use of a
bacteriostatic antibiotic. When a bacteriostatic antibiotic is used the duration
of therapy must be sufficient to allow cellular and humoral defense mechanisms
to eradicate the bacteria. If possible, bactericidal antibiotics should be used
to treat infections of the
endocardium or the
meninges. Host defenses
are relatively ineffective at these sites and the dangers imposed by such infections
require prompt eradication of the organisms.
Antibiotic Susceptibility
Testing
The basic quantitative
measures of the in vitro activity of antibiotics are the minimum
inhibitory concentration (MIC) and the minimum bactericidal
concentration (MBC). The MIC is the lowest concentration of the
antibiotic that results in inhibition of visible growth (i.e. colonies on
a plate or turbidity in broth culture) under standard conditions. The MBC is the
lowest concentration of the antibiotic that kills 99.9% of the original inoculum
in a given time. Figure 1 illustrates how to determine the MIC of an antibiotic.
|
 Fig 1 Antibiotic susceptibility
testing
Fig 1 Antibiotic susceptibility
testing |
KEY WORDS
Selectivity
Therapeutic
Index
Bactericidal
Bacteriostatic
MIC
MBC
Disk Diffusion
Test
Antibiotic
Synergism
Antibiotic
Antagonism
Antimicrobial
Cross
Resistance
Multiple Resistance |
For an antibiotic to be effective the MIC or MBC must be able
to be achieved at the site of the infection. The pharmacological absorption and
distribution of the antibiotic will influence the dose, route and frequency of
administration of the antibiotic in order to achieve an effective dose at the
site of infection.
In clinical laboratories, a more common test for antibiotic
susceptibility is a disk diffusion test (figure 1). In this test the bacterial isolate is
inoculated uniformly onto the surface of an agar plate. A filter disk
impregnated with a standard amount of an antibiotic is applied to the surface of
the plate and the antibiotic is allowed to diffuse into the adjacent medium..
The result is a gradient of antibiotic surrounding the disk. Following
incubation, a bacterial lawn appears on the plate. Zones of inhibition of
bacterial growth may be present around the antibiotic disk. The size of the zone
of inhibition is dependent on the diffusion rate of the antibiotic, the degree
of sensitivity of the microorganism, and the growth rate of the bacterium. The
zone of inhibition in the disk diffusion test is inversely related to the MIC.
The test is performed under standardized conditions and
standard zones of inhibition have been established for each antibiotic. If the
zone of inhibition is equal to or greater than the standard, the organism is
considered to be sensitive to the antibiotic. If the zone of inhibition is less
than the standard, the organism is considered to be resistant. Figure 1 also
illustrates how the disk diffusion test is done and Figure 2 illustrates some of
the standard zones of inhibition for several antibiotics.
Combination Therapy
Combination therapy with two or more
antibiotics is used in special cases:
-
To prevent the emergence of resistant
strains
-
To treat emergency cases during the period when an etiological
diagnosis is still in progress
-
To take advantage of antibiotic
synergism.
Antibiotic synergism occurs when the effects of a
combination of antibiotics is greater than the sum of the effects of the
individual antibiotics. Antibiotic antagonism occurs when one
antibiotic, usually the one with the least effect, interferes with the effects
of another antibiotic.
Antibiotics and
Chemotherapeutic agents
The term antibiotic
strictly refers to substances that are of biological origin whereas the term chemotherapeutic
agent refers to a synthetic chemical. The distinction between these
terms has been blurred because many of our newer "antibiotics"
are actually chemically modified biological products or even chemically
synthesized biological products. The generic terms to refer to either
antibiotics or chemotherapeutic agents are antimicrobic or antimicrobial
agent. However, the term antibiotic is often used to refer to all types
of antimicrobial agents.
|
| |
|
Figure 2
Zone diameter interpretive standards and approximate MIC
correlates
used to define the interpretive categories
|
|
Antimicrobial agent
(amount per disk)
and organism |
Zone diameter (nearest whole millimeter) for each
interpretive category |
|
Approximate MIC correlates (micro gm/ml) for:
|
|
R |
I |
MS |
S |
|
R |
S |
|
Ampicillin (10 micro gm) |
|
|
|
|
|
|
|
Enterobacteriaceae
|
<11 |
12-13 |
|
>14 |
|
>32 |
<8 |
Staphylococcus
spp.
|
<28 |
|
|
>29 |
|
beta-Lactamase |
<0.25 |
Haemophilus
spp.
|
<19 |
|
|
>20 |
|
>4 |
<2 |
Enterococci
|
<16 |
|
>17 |
|
|
>16 |
|
Other streptococci
|
<21 |
|
22-29 |
>30 |
|
>4 |
<0.12 |
|
Chloramphenicol (30 micro gm) |
<12 |
13-17 |
|
>18 |
|
>25 |
<12.5 |
|
Erythromycin (15 micro gm) |
<13 |
14-17 |
|
>18 |
|
>8 |
<2 |
|
Nalidixic acid (30 micro gm) |
<13 |
14-18 |
|
>19 |
|
>32 |
<12 |
|
Streptomycin (10 micro gm |
<11 |
12-14 |
|
>15 |
|
|
|
|
Tetracycline (30 micro gm) |
<14 |
15-18 |
|
>19 |
|
>16 |
<4 |
|
Trimethoprim (5 micro gm) |
<10 |
11-15 |
|
>16 |
|
>16 |
<4 |
a
Adapted from the October 1983 document (M2-T3)
of the NCCLS. Refer to the most current MCCLS documents for updates and
changes.
b
R, Resistant; I, intermediate; MS, moderately
susceptible; S, susceptible. An I result should be reported since it
indicates an equivocal test result that may require further testing.
When designated in the table, an MS result should be reported to
indicate a level of susceptibility that should require the maximal safe
dosage for therapy. Strains in the MS category are susceptible and not
intermediate.
c
Approximate MIC correlates used for the
definition of the resistant and susceptible categories. Theses
correlates should not be used for the interpretation of antimicrobial
dilution test results.
|
|
|
|
Protein Synthesis and Site of Action of
Antimicrobials that Inhibit Protein Synthesis
Initiation of Protein Synthesis
Figure 3 illustrates the
initiation of protein synthesis and the site of action of antimicrobials that
inhibit this process.
Elongation
Figure 4 illustrates the process of elongation
and the site of action of antimicrobials that inhibit this process.
Inhibitors of Protein Synthesis
The selectivity of these agents is a result of differences in
the prokaryotic 70S ribosome and the 80S eukaryotic ribosome. Since
mitochondrial ribosomes are similar to prokaryotic ribosomes, these antimetabolites can have some toxicity.
They are mostly bacteriostatic.
Antimicrobials that Bind to the 30S Ribosomal Subunit
Aminoglycosides
(bactericidal)
Streptomycin, kanamycin, gentamicin, tobramycin, amikacin, netilmicin and neomycin (topical)
a. Mode of action
The aminoglycosides irreversibly bind to the
30S ribosome and freeze the 30S initiation complex (30S-mRNA-tRNA), so that no
further initiation can occur. The aminoglycosides also slow down protein
synthesis that has already initiated and induce misreading of the mRNA.
b. Spectrum of
Activity
Aminoglycosides are active against many gram-negative and some
gram-positive bacteria. They are not useful for anaerobic bacteria, since oxygen
is required for uptake of
the antibiotic, or for intracellular bacteria.
c. Resistance
Resistance to these antibiotics is common
d. Synergy
The aminoglycosides synergize with β-lactam
antibiotics such as the penicillins. The β-lactams inhibit cell wall synthesis and thereby increase
the permeability of the bacterium to the aminoglycosides.
|
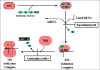 Fig 3 Antibiotics that act at the level of
protein synthesis initiation
Fig 3 Antibiotics that act at the level of
protein synthesis initiation
 Fig 4 Antibiotics that act at the level
of the elongation phase of protein synthesis
Fig 4 Antibiotics that act at the level
of the elongation phase of protein synthesis
 Streptomycin
Streptomycin
Neomycin |
Tetracycline
Spectinolycin |
Tetracyclines
(bacteriostatic)
Tetracycline, minocycline and doxycycline
a. Mode of action
The tetracyclines reversibly bind to the 30S
ribosome and inhibit binding of aminoacyl-t-RNA to the acceptor site on the 70S
ribosome.
b. Spectrum of activity -
These are broad spectrum antibiotics and are useful against
intracellular bacteria
c. Resistance
Resistance to these antibiotics is common
d. Adverse effects
Destruction of normal intestinal flora often occurs,
resulting in increased secondary infections. There can also be staining and impairment of the
structure of bone and teeth
Spectinomycin
(bacteriostatic)
a. Mode of action
Spectinomycin reversibly interferes with
mRNA interaction with the 30S ribosome. It is structurally similar
to aminoglycosides but does not cause misreading of mRNA
b. Spectrum of activity -
Spectinomycin is used in the treatment of
penicillin-resistant Neisseria gonorrhoeae
c. Resistance
This is rare in
Neisseria gonorrhoeae
|
Chloramphenicol
Erythromycin
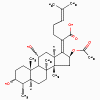
Fusidic acid
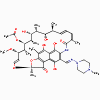
Rifampin
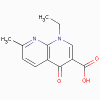
Nalidixic acid
|
Antimicrobials that Bind to the 50S Ribosomal Subunit
Chloramphenicol, lincomycin, clindamycin
(bacteriostatic)
a. Mode of action
These antimicrobials bind to the 50S
ribosome and inhibit peptidyl transferase activity.
b. Spectrum of activity
c. Resistance
Resistance to these antibiotics is common
d. Adverse effects
Chloramphenicol is toxic (bone marrow
suppression) but it is used in the treatment of bacterial meningitis.
Macrolides
(bacteriostatic) - Erythromycin (also azithromycin,
clarithromycin)
a. Mode of action
The macrolides inhibit translocation of the peptidyl tRNA from the A to
the P site on the ribosome by binding to the 50S ribosomal 23S RNA.
b. Spectrum of
activity
Gram-positive bacteria, Mycoplasma,
Legionella
c. Resistance
Resistance to these antibiotics is common. Most gram-negative
antibiotics are resistant to macrolides.
Antimicrobials that Interfere with Elongation Factors
Fusidic acid
(bacteriostatic)
a. Mode of action
Fusidic acid binds to elongation factor G (EF-G)
and inhibits release of EF-G from the EF-G/GDP complex.
b. Spectrum of
activity
Fusidic acid is only effective against gram-positive bacteria such as
Streptococcus, Staphylococcus aureus and Corynebacterium
minutissimum.
Inhibitors of Nucleic Acid Synthesis and Function
The selectivity of these agents is a result of differences in prokaryotic and
eukaryotic enzymes affected by the antimicrobial agent.
Inhibitors of RNA Synthesis and Function
Rifampin, rifamycin, rifampicin
(bactericidal)
a. Mode of action
These antimicrobials bind to DNA-dependent
RNA polymerase and inhibit initiation of RNA synthesis.
b. Spectrum of
activity
They are wide spectrum antibiotics but are used most
commonly in the treatment of tuberculosis
c. Resistance
Resistance to these antibiotic is common.
d. Combination therapy
Since resistance is common, rifampin is
usually used in combination therapy
Inhibitors of DNA Synthesis and Function
Quinolones
- nalidixic acid, ciprofloxacin,
oxolinic acid
(bactericidal)
a. Mode of action
These antimicrobials bind to the A subunit
of DNA gyrase (topoisomerase) and prevent supercoiling of DNA, thereby
inhibiting DNA synthesis.
b. Spectrum of activity -
These antibiotics are active against Gram-positive cocci and are used in urinary tract
infections
c. Resistance
This is common for nalidixic acid and is developing for
ciprofloxacin
|
|
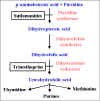 Fig 5 Folic acid metabolism
Fig 5 Folic acid metabolism
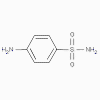
Sulfanilamide
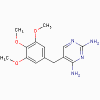
Trimethoprim
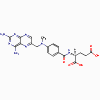
Methotrexate
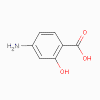
Amino salicylic acid
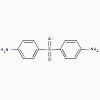
Dapsone
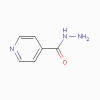
Isoniazid |
Antimetabolite Antimicrobials
Inhibitors of Folic Acid Synthesis
The selectivity
of these antimicrobials is a consequence of the fact that bacteria cannot use
pre-formed folic acid and must synthesize their own folic acid. In contrast,
mammalian cells use folic acid obtained from food.
Figure 5 summarizes the
pathway of folic acid metabolism and indicates the sites at which antimetabolites act.
Sulfonamides, sulfones
(bacteriostatic)
a. Mode of action
These antimicrobials are analogues of para-aminobenzoic acid and competitively inhibit formation of dihydropteric
acid.
b. Spectrum of
activity
They have a broad range activity against
gram-positive and gram-negative bacteria and are used primarily in urinary tract
infections and in Nocardia infections.
c. Resistance
Resistance to these antibiotics is common
d. Combination
therapy
The sulfonamides are used in
combination with trimethoprim. This combination blocks two distinct steps in
folic acid metabolism and prevents the emergence of resistant strains.
Trimethoprim,
methotrexate, pyrimethamine (bacteriostatic)
a. Mode of action
These antimicrobials bind to dihydrofolate
reductase and inhibit formation of tetrahydrofolic acid.
b. Spectrum of
activity
They have a broad range activity against
gram-positive and gram-negative bacteria and are used primarily in urinary tract
infections and in Nocardia infections.
c. Resistance
Resistance to these antibiotics is common
d. Combination
therapy
These antimicrobials are used in
combination with the sulfonamides. This combination blocks two distinct steps in
folic acid metabolism and prevents the emergence of resistant strains.
Anti-Mycobacterial agents
Anti-mycobacterial agents
are generally used in combination with other antimicrobials since treatment is
prolonged and resistance develops readily to individual agents.
Para-aminosalicylic acid (PSA)
(bacteriostatic)
a. Mode of action
This is similar to sulfonamides
b. Spectrum of
activity
PSA is specific for Mycobacterium
tuberculosis
Dapsone
(bacteriostatic)
a. Mode of action
Similar to sulfonamides
b. Spectrum of
activity
Dapsone is used in treatment of leprosy
Isoniazid (INH)
(bacteriostatic)
a. Mode of action
Isoniazid inhibit synthesis of mycolic
acids.
b. Spectrum of activity -
INH is used in treatment of tuberculosis
c. Resistance
Resistance has developed
|
| |
Antimicrobial Drug Resistance
Principles and Definitions
Clinical Resistance
Clinical resistance to an antimicrobial agent occurs when the
MIC of the drug for a particular strain of bacteria exceeds that which is
capable of being achieved with safety in vivo. Resistance to an
antimicrobial can arise:
Resistance that appears after
introduction of an antimicrobial agent into the environment usually results from
a selective process, i.e. the antibiotic selects for survival of those strains
possessing a resistance gene. Resistance can develop in a single step or it can
result from the accumulation of multiple mutations.
Cross Resistance
Cross resistance implies that a single
mechanism confers resistance to multiple antimicrobial agents while multiple
resistance implies that multiple mechanisms are involved. Cross
resistance is commonly seen with closely related antimicrobial agents while
multiple resistance is seen with unrelated antimicrobial agents.
Mechanisms of Resistance
Altered
permeability of the antimicrobial agent
Altered
permeability may be due to the inability of the antimicrobial agent to enter the
bacterial cell or alternatively to the active export of the agent from the cell.
Inactivation of the
antimicrobial agent
Resistance is often
the result of the production of an enzyme that is capable of inactivating the
antimicrobial agent.
Altered target site
Resistance can arise due to alteration
of the target site for the antimicrobial agent.
Replacement of a
sensitive pathway
Resistance can result
from the acquisition of a new enzyme to replace the sensitive one.
|
|
|
 Return to the Bacteriology section of Microbiology and Immunology On-line
Return to the Bacteriology section of Microbiology and Immunology On-line
This page last changed on
Friday, February 26, 2016
Page maintained by
Richard Hunt
|

 Fig 1 Antibiotic susceptibility
testing
Fig 1 Antibiotic susceptibility
testing Fig 3 Antibiotics that act at the level of
protein synthesis initiation
Fig 3 Antibiotics that act at the level of
protein synthesis initiation

















