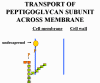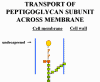| x | x | ||||
 |
|||||
| BACTERIOLOGY | IMMUNOLOGY | MYCOLOGY | PARASITOLOGY | VIROLOGY | |
|
|
|
||||
|
|
|||||
| ALBANIAN | |||||
| FARSI | |||||
|
Let us know what you think FEEDBACK |
|||||
| SEARCH | |||||
|
|
|||||
| Logo image © Jeffrey Nelson, Rush University, Chicago, Illinois and The MicrobeLibrary | |||||
|
|
|||||
|
KEY WORDS |
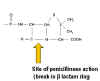
STERILIZATION Sterilization refers to killing (or removal) of ALL bacteria in a non-selective fashion. For example, autoclaving involves heating liquids (e.g. media) or solids to 121oC under steam pressure. The materials must be heat resistant. Ethylene oxide is sometimes used in hospitals for equipment that cannot be heated. Membrane filters have pores that trap bacteria, but allow drugs and small chemicals to pass through; thus pre-sterilized filters can be used to sterilize delicate solutions. UV light is used for decreasing bacterial levels on surfaces such as in operating rooms; however it is not totally effective. Ionizing radiation is more efficient and can be used for sterilizing instruments and food. Disinfectants (e.g. phenol-based) can be useful in killing many bacteria on certain instruments, but cannot be used for internal consumption or on skin. Antiseptics (e.g. iodine or 70% alcohol) are used topically (e.g. on skin surfaces) to reduce bacterial load. ANTIBIOTICS In contrast, antibiotics are agents that are "selectively" toxic for bacteria (either killing them [bactericidal] or inhibiting their growth [bacteriostatic]) without harm to the patient. They can thus be ingested. By definition, these compounds must act on structures found in bacteria, but not in the host. Antibiotics work most efficiently in conjunction with an active immune system to kill infecting bacteria in the host. After isolation of pure colonies (see Bacteriology lecture 2), the susceptibility of bacterial isolates can be tested to a variety of antibiotics. The minimal inhibitory concentration (MIC) refers to the lowest concentration of an antibiotic that stops visible growth. More simply, the zone of inhibition around a disk impregnated with antibiotic (Kirby-Bauer) is another measure of antibiotic activity. INHIBITORS OF CELL WALL SYNTHESIS One major class of antibiotics inhibits the synthesis of peptidoglycan (figure 1). Once cell wall synthesis (involving penicillin binding proteins) is inhibited, enzymatic autolysis of the cell wall can occur. Without the restraining influence of the cell wall the high osmotic pressure inside the cell bursts the inner and/or outer membranes of bacteria. Thus, these antibiotics are generally bactericidal. Several mechanisms are involved in inhibition of peptidoglycan synthesis:
|
||||
|
TUTORIAL |
|||||
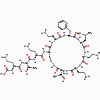
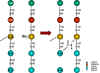 Figure 1 Cross-linking of peptidoglycan
Figure 1 Cross-linking of peptidoglycan
|
|
||||
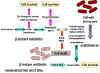 Figure 3 Resistance to beta
lactams - Gram-negative bacteria
Figure 3 Resistance to beta
lactams - Gram-negative bacteria Figure 4 Resistance to beta
lactams - Gram-positive bacteria
Figure 4 Resistance to beta
lactams - Gram-positive bacteria |
PENICILLIN Penicillin is made by the mold Penicillium chrysogenum. During fermentation the mold forms 6-aminopenicillanic acid which has a thiazolidine ring and a beta-lactam ring fused together (figure 2). This, however, is acid labile and subject to degradation by bacterial enzymes. More stable derivatives are made biochemically so that in addition to increased stability, they are better absorbed from the gastro-intestinal tract and have a wider spectrum of bactericidal effects. Various chemical side chains have been synthetically linked to the ring structures producing a host of antibiotics with different properties in the host. Many penicillins (figure 2) display little activity against Gram negative bacteria, since they do not penetrate the outer membrane. Cephalosporins and other newer penicillins are active against Gram negative bacteria, since they can penetrate the outer membrane. Other chemically modified penicillins have lower elimination rates from the patient; decreasing the frequency of administration of these drugs. Penicillins can be destroyed by beta lactamase (penicillinase)
produced by resistant bacterial strains (figure 3). Clavulinic acid, also has a beta lactam
component which binds strongly to beta lactamases inhibiting their activity. It
is used in conjunction with certain penicillins allowing their use against
otherwise resistant bacteria. Another form of resistance involves a change in
the structure of penicillin binding proteins such that the antibiotic does not
bind efficiently (figure 4). In the case of Gram negative bacteria, penicillins pass across
the outer membrane using porins. Resistance may develop from mutation leading to
modified porins.
|
||||

|
POLYMYXIN B Polymyxin B (figure 5) binds to the lipid A portion of lipopolysaccharide and also to phospholipids. However, it binds preferentially to lipid A. This disrupts the outer membrane of Gram negative bacteria. Since the cell membrane is not exposed in Gram positive bacteria polymyxin has little activity against them. This drug is toxic to human cells, since it can also lyze eukaryotic membranes; this explains its limited clinical use. |
||||
 Figure 5A
Figure 5AVancomycin |
VANCOMYCIN Vancomycin is a drug of last resort against Gram-positive bacteria. It is a glycopeptide (figure 5A) made by an Acinobacter species. Vancomycin-resistance has arisen making this antibiotic less useful. It is very hydrophilic and forms hydrogen binds with terminal D-alanyl-D-alanine moieties of the NAM/NAG-subunits and stops polymerization of the subunits to form long chains. It also prevents polymer cross-linking. Vancomycin use is often replaced by Daptomycin.
|
||||
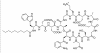 Figure 6 Daptomycin (Cubicin)
Figure 6 Daptomycin (Cubicin) |
DAPTOMYCIN Daptomycin (Cubicin - figure 6) is a natural lipopeptide that is used to treat multi-resistant Gram-positive bacterial infections. It is a natural product made by the soil fungus Streptomyces roseosporus. The lipid portion of the molecule binds to the cell membrane resulting in depolarization (loss of membrane potential). It can be used to treat:
It is used in the United States against Gram-positive skin infections, Staphylococcus aureus bacteremia and right-sided Staphylococcus aureus endocarditis. Daptomycin cannot be used to treat pneumonia because it binds to pulmonary surfactant. Daptomycin may cause life-threatening eosinophilic pneumonia in people over
60 years. |
||||
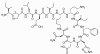 Figure 7
Figure 7Bacttracin |
BACITRACIN Bacitracin is a cyclic polypeptide produced by Bacillus subtilis var Tracy. It is used topically against Gram-positive bacterial eye and skin infections but is not used systemically. Bacitracin inhibits dephosphorylation of C55-isoprenyl pyrophosphate which transports peptidoglycan components bacterial cell walls outside the inner membrane |
||||
|
Transport of a peptidoglycan subunit across the cell membrane to the cell wall
|
|||||
|
|
|
||||