| x | x | |||||||
 |
||||||||
| BACTERIOLOGY | IMMUNOLOGY | MYCOLOGY | PARASITOLOGY | VIROLOGY | ||||
|
||||||||
| En Espaņol | ||||||||
| SHQIP - ALBANIAN | ||||||||
|
Let us know what you think FEEDBACK |
||||||||
| SEARCH | ||||||||
|
|
||||||||
|
THIS CHAPTER IS
IN SEVERAL PARTS |
||||||||
|
LINKS TO OTHER HIV AND AIDS SECTIONS ARE AT THE BOTTOM OF THIS PAGE |
||||||||
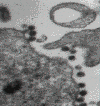
Figure 15
|
Cells that are infected by HIV HIV destroys CD4+ T4 cells specifically, causing the profound immuno-suppression that is the hallmark of AIDS. Some other cells harbor and replicate the virus without lysis or, in the case of dendritic cells, they may concentrate virus at the cell surface with little or no replication of the virus. The major HIV-infected cell types are shown in figure 15c.
|
|||||||
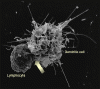 Figure 15b
Figure 15bThe interaction of a dendritic cell (right) with a lymphocyte (left). HIV bound to the surface of the dendritic cell is clustered at the site of interaction between the two cells (arrow), thereby facilitating the infection of the lymphocyte. On T4 cells, HIV receptors also concentrate here Steve Haley - William Bowers - Richard Hunt
|
||||||||
|
The necessity for CD4 antigen expression for entry of HIV into a human cell. HeLa cells do not have CD4 antigen and are not infected. HeLa cells transfected with CD4 gene are infected
|
CD4 antigen is the HIV receptor The apparent specificity of CD4+ cell infection observed early in the history of HIV and AIDS, together with the observation that T4 cells are depleted in disease (indeed, the course of disease in the patient is followed by CD4+ T cell levels), suggested that CD4 antigen might be the receptor for the virus. This was demonstrated by transfecting the CD4 antigen gene into CD4- human cells (such as cervical carcinoma HeLa cells) and showing that they acquired the property of being able to be infected by HIV (figure 16). CD4 antigen is the major receptor for both HIV-1 and HIV-2 in T4 cells and most other cells that can be infected by HIV.
|
|||||||
|
A co-receptor for infection by HIV The experiment in which CD4 antigen is transfected into cells that then acquire the ability to be infected by HIV only works when the transfected cells are human. If we do the same experiment with mouse 3T3 cells, the virus can bind to the cell surface, via CD4 antigen, but no infection ensues. Thus, something more than CD4 antigen is necessary. It was also discovered that some strains of HIV (those adapted for life in transformed T cells) could infect and replicate in activated human T cells but not in monocytes or macrophages. Conversely, those adapted for life in macrophages could not infect and replicate in transformed T cells. Yet both macrophages and T4 cells possess CD4 antigen. The differences in tropism of the viral strains mapped to the V3 region of Gp120 suggesting that molecules other than CD4 antigen have an important role in infection and this role is CD4+ cell type-specific.
|
||||||||
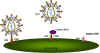 Figure 17A
Figure 17AChemokine receptors are involved, in association with CD4 antigen, in infection by HIV (left). The chemokine can block attachment of the virus to its receptors (middle). Mutations in the chemokine receptor can lead to resistance to HIV infection (right)
|
It was originally thought that only cells that have CD4 antigen can be infected by HIV. Although CD4 protein had not been demonstrated on some infectable cells, it was thought to be present in low amounts and CD4 antigen mRNA could be detected in most infectable cells. Specificity to CD4 positive cells reflects the specific binding of Gp120 to CD4. It is now known, however, that some non-CD4 cells, including some in brain and intestine, can be infected in a via a galactocerebroside receptor. Other cells can be infected in a different way; for example, in macrophages (see below) an Fc or complement receptor may be used. In these cases, the HIV must be bound by anti-HIV antibodies that interact with receptors on the cell surface. Thus anything that can up-regulate Fc receptors on macrophages will augment infection.
Entry into cell: pH-independent fusion with plasma membrane No pH-dependent conformational change in a viral membrane protein is necessary for fusion between the viral membrane and the membrane of the cell to be infected. Thus, no entry into endosomes or lysosomes is required. As with herpes virus, this sort of fusion of a virus with the plasma membrane is associated with fusions of infected cells to form syncytia. Syncytium formation is also a characteristic of HIV infection (figure 18). This has profound significance for spread of infection between cells without any free virus. This means that virus may spread from cell to cell so that immune system circulatory antibodies cannot have any effect (problem for vaccine). Not only will a vaccine have to be able to destroy the virus, it will also have to be able to destroy infected cells. Gp41 is the fusogen. Syncytia are most often seen in brain.
Reverse transcription and integration This is similar to other retroviruses. HIV uses reverse transcriptase imported during infection as part of the virus. The nucleocapsid enters the cytoplasm and reverse transcription occurs within the nucleocapsid. In naive resting T4 cells, the provirus (DNA form) remains in the cytoplasm, possibly because of the lack of ATP necessary for the energy-dependent import of the pre-integration complex into the nucleus. Most viruses that replicate in the nucleus can do so only in dividing cells but cell division is not essential for HIV replication. This is because two viral proteins (Vpr and one of the GAG proteins) have nuclear localization signals and so nuclear membrane breakdown at mitosis, which allows penetration of viral DNA to the chromosomes, is not necessary.
Integration
After uncoating and entry into
the nucleus, both linear and circular forms of the viral DNA are found.
Linear double strand viral DNA is inserted into the host cell
chromosomes using the viral integrase protein (translated from the pol
gene). After integration, viral RNA is transcribed by host RNA
polymerase II.
|
|||||||
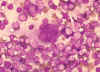 Figure 18
Figure 18Multinucleated cell (syncytium) in touch preparation from cut surface of enlarged lymph node from patient with HIV-1 infection. Cell fusion producing a large multinucleated cell is a viral cytopathic effect characteristic, but not diagnostic, of infection by HIV-1. Giemsa stain. Lymphadenopathy smear. CDC/Dr. Edwin P. Ewing, Jr. epe1@cdc.gov
|
||||||||
| Figure 19
|
Assembly of new virus takes place at the membrane of the host cell (figure 19). Three types of protein make up the virion. These are the membrane protein complex (Gp120 and Gp41 - originally derived from Gp160) plus two internal precursor proteins, the Gag polyprotein and the Gag/Pol polyprotein (the latter is the result of a frame shift that allows the ribosome to continue translation from the Gag gene into the Pol gene) The proteins aggregate at the cell membrane and the membrane pinches off (figure 19 and 20). The larger internal precursor (Gag-Pol) draws two strands of the positive strand RNA into the nascent virion and the protease (part of the Gag-Pol protein) cuts itself free. The protease completes the cleavage of Gag-Pol to liberate other enzymes (reverse transcriptase, integrase and more protease). The protease also cleaves the remainder of Gag-Pol and the smaller Gag into structural proteins. p24, p7 and p6 form the bullet-shaped core while p24 underlies the membrane. The Gag and Gag/Pol fusion proteins are made in ratio of about 20:1. This specific viral protease is vital as the viral proteins are not functional unless separated. This specificity makes the protease a good candidate for inhibition by anti-HIV drugs (see appendix 3 and anti-viral chemotherapy). Gag/Pol and Gag are attached to the viral membrane via a fatty acid that is covalently bound. The cleavages result in p17 remaining attached to the membrane. Gp160 is translated from a singly spliced mRNA in association with the endoplasmic reticulum and is an integral membrane protein that spans the membrane once. In the endoplasmic reticulum, it is glycosylated before being transferred to the Golgi Body where it is further glycosylated and cleaved by a host enzyme to gp120 and gp41. It moves to the cell membrane via the exocytic pathway. In contrast to Gag and Gag-Pol proteins, gp160 is not cleaved by the viral protease.
|
|||||||
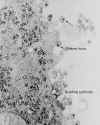 Figure 20
Figure 20Transmission electron micrograph of HIV-1, budding and free CDC
|
||||||||
|
||||||||
|
||||||||