|
x |
x |
|
 |
 |
|
INFECTIOUS
DISEASE |
BACTERIOLOGY |
IMMUNOLOGY |
MYCOLOGY |
PARASITOLOGY |
VIROLOGY |
|
SLOVAK
|
IMMUNOLOGY - CHAPTER ONE
INNATE
(NON-SPECIFIC) IMMUNITY
Gene Mayer, Ph.D
Emertius Professor of Pathology, Microbiology and Immunology
University of South Carolina
|
|
VIETNAMESE |
|
FRENCH |
|
SPANISH |
|
PORTUGUESE |
|
ALBANIAN |
|
TURKISH |
|
Let us know what you think
FEEDBACK |
|
SEARCH |
|
|
|
 |
|
Logo image
© Jeffrey Nelson, Rush University, Chicago, Illinois and
The MicrobeLibrary |
|
TEACHING OBJECTIVES
Recognize the significance of the immune system in
combating infection and disease
Distinguish between non-specific (innate) and
specific (adaptive) immune systems
Understand the mechanisms combating infection/disease
(killing pathogens)
Know the humoral and cellular components of the
non-specific immunity
Comprehend the mechanism of action of
the humoral and
cellular components of non-specific immunity
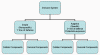 Figure 1
Figure 1
Overview of the immune system
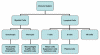 Figure 2
Figure 2
Cells of the immune system
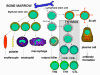 Figure 3
Figure 3
Development of the cells of the immune system |
OVERVIEW OF THE IMMUNE SYSTEM
We are constantly being exposed to infectious agents and yet, in most
cases, we are able to resist these infections. It is our immune system
that enables us to resist infections. The immune system is composed of
two major subdivisions, the innate or non-specific immune system and the
adaptive or specific immune system (Figure 1). The innate immune system
is our first line of defense against invading organisms while the
adaptive immune system acts as a second line of defense and also affords
protection against re-exposure to the same pathogen. Each of the major
subdivisions of the immune system has both cellular and
humoral
components by which they carry out their protective function (Figure 1).
In addition, the innate immune system also has anatomical features that
function as barriers to infection. Although these two arms of the immune
system have distinct functions, there is interplay between these systems
(i.e., components of the innate immune system influence the adaptive
immune system and vice versa).
Although the innate and adaptive immune systems both function to protect
against invading organisms, they differ in a number of ways. The
adaptive immune system requires some time to react to an invading
organism, whereas the innate immune system includes defenses that, for
the most part, are constitutively present and ready to be mobilized upon
infection. Second, the adaptive immune system is antigen specific and
reacts only with the organism that induced the response. In contrast,
the innate system is not antigen specific and reacts equally well to a
variety of organisms. Finally, the adaptive immune system demonstrates
immunological memory. It “remembers” that it has encountered an invading
organism and reacts more rapidly on subsequent exposure to the same
organism. In contrast, the innate immune system does not demonstrate
immunological memory.
All cells of the immune system have their origin in the bone marrow and
they include
myeloid (neutrophils, basophils, eosinpophils, macrophages
and dendritic cells) and lymphoid (B lymphocyte, T lymphocyte and
Natural Killer) cells (Figure 2), which differentiate along distinct
pathways (Figure 3). The myeloid progenitor (stem) cell in the bone
marrow gives rise to erythrocytes, platelets, neutrophils, monocytes/macrophages
and dendritic cells whereas the lymphoid progenitor (stem) cell gives
rise to the NK, T cells and B cells. For T cell development the
precursor T cells must migrate to the thymus where they undergo
differentiation into two distinct types of T cells, the CD4+ T helper
cell and the CD8+ pre-cytotoxic T cell. Two types of T helper cells are
produced in the thymus the TH1 cells, which help the CD8+ pre-cytotoxic
cells to differentiate into cytotoxic T cells, and TH2 cells, which help
B cells, differentiate into plasma cells, which secrete antibodies.
The main function of the immune system is self/non-self discrimination.
This ability to distinguish between self and non-self is necessary to
protect the organism from invading pathogens and to eliminate modified
or altered cells (e.g. malignant cells). Since pathogens may replicate
intracellularly (viruses and some bacteria and parasites) or
extracellularly (most bacteria, fungi and parasites), different
components of the immune system have evolved to protect against these
different types of pathogens. It is important to remember that infection
with an organism does not necessarily mean diseases, since the immune
system in most cases will be able to eliminate the infection before
disease occurs. Disease occurs only when the bolus of infection is high,
when the virulence of the invading organism is great or when immunity is
compromised. Although the immune system, for the most part, has
beneficial effects, there can be detrimental effects as well. During
inflammation, which is the response to an invading organism, there may
be local discomfort and collateral damage to healthy tissue as a result
of the toxic products produced by the immune response. In addition, in
some cases the immune response can be directed toward self tissues
resulting in autoimmune disease.
|
Table 1 |
|
Non-specific Immunity |
Specific Immunity |
| Response is antigen-independent |
Response is antigen-dependent |
|
There is immediate maximal response |
There is a lag time between exposure and maximal response |
| Not antigen-specific |
Antigen-specific |
|
Exposure results in no immunologic memory |
Exposure results in immunologic memory |
|
| |
INNATE (NON-SPECIFIC) IMMUNITY
The elements of the innate (non-specific) immune system (Table 2) include
anatomical barriers, secretory molecules and cellular components. Among the
mechanical anatomical barriers are the skin and internal epithelial layers,
the movement of the intestines and the oscillation
of broncho-pulmonary
cilia. Associated with these protective surfaces are
chemical and biological agents.
Anatomical barriers to infections
Mechanical factors
The epithelial surfaces form a physical barrier that is very impermeable to most
infectious agents. Thus, the skin acts as our first line of defense against
invading organisms. The desquamation of skin epithelium also helps remove
bacteria and other infectious agents that have adhered to the epithelial
surfaces. Movement due to cilia or peristalsis helps to keep air passages and
the gastrointestinal tract free from microorganisms. The flushing action of
tears and saliva helps prevent infection of the eyes and mouth. The trapping
effect of mucus that lines the respiratory and gastrointestinal tract helps
protect the lungs and digestive systems from infection.
Chemical factors
Fatty acids in sweat inhibit the growth of bacteria.
Lysozyme and
phospholipase
found in tears, saliva and nasal secretions can breakdown the cell wall of
bacteria and destabilize bacterial membranes. The low pH of sweat and gastric
secretions prevents growth of bacteria.
Defensins (low molecular weight
proteins) found in the lung and gastrointestinal tract have antimicrobial
activity. Sweat also contains low molecular weight anti-microbial peptides that
interact with bacterial cell membranes (including MRSA) in which they form a
channel that allows the passage of water and ions, disrupting the
transmembrane potential, leading to the death of the cell.
Surfactants in the lung act as opsonins (substances that promote
phagocytosis of particles by phagocytic cells).
Biological factors
The normal flora of the skin and in the gastrointestinal tract can prevent the
colonization of pathogenic bacteria by secreting toxic substances or by
competing with pathogenic bacteria for nutrients or attachment to cell surfaces.
Humoral barriers to infection
The anatomical barriers are very effective in preventing colonization of tissues
by microorganisms. However, when there is damage to tissues the anatomical
barriers are breached and infection may occur. Once infectious agents have
penetrated tissues, another innate defense mechanism comes into play, namely
acute inflammation. Humoral factors play an important role in inflammation,
which is characterized by
edema and the
recruitment of
phagocytic cells. These humoral factors are found in serum or they are
formed at the site of infection.
Complement system
The complement system is the major humoral non-specific defense mechanism (see
complement chapter). Once activated complement can lead to increased
vascular permeability, recruitment of phagocytic cells, and lysis and
opsonization of bacteria.
Coagulation system
Depending on the severity of the tissue injury, the
coagulation system may or may not be activated. Some products of the
coagulation system can contribute to the non-specific defenses because of
their ability to increase vascular permeability and act as
chemotactic agents for phagocytic cells. In addition, some of the
products of the coagulation system are directly antimicrobial. For example,
beta-lysin, a protein produced by platelets during coagulation can lyse many
Gram positive bacteria by acting as a cationic detergent.
Lactoferrin and transferrin
By binding iron, an essential nutrient for
bacteria, these proteins limit bacterial growth.
Interferons
Interferons are proteins that can limit virus replication
in cells.
Lysozyme
Lysozyme breaks down the cell wall of bacteria.
Interleukin-1
Il-1 induces fever and the production of acute phase
proteins, some of which are antimicrobial because they can opsonize
bacteria.
|
 Figure 4A
Two neutrophils in blood film © Bristol Biomedical Image Archive
Used with permission
Figure 4A
Two neutrophils in blood film © Bristol Biomedical Image Archive
Used with permission
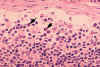 Figure 4B Histopathology of lymphadenopathy due to infection by HIV-1. Subcapsular sinus. The
sinus contains increased numbers of neutrophils. CDC/Dr. Edwin P. Ewing, Jr. epe1@cdc.gov
Figure 4B Histopathology of lymphadenopathy due to infection by HIV-1. Subcapsular sinus. The
sinus contains increased numbers of neutrophils. CDC/Dr. Edwin P. Ewing, Jr. epe1@cdc.gov
 Figure 4C
Figure 4C
Neutrophil - electron micrograph. Note the two nuclear lobes and the
azurophilic granules © Dr Louise
Odor, University of South Carolina School of Medicine
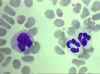 Figure 4D
Blood film showing a monocyte (left) and two neutrophils © Bristol Biomedical Image Archive
Used with permission
Figure 4D
Blood film showing a monocyte (left) and two neutrophils © Bristol Biomedical Image Archive
Used with permission
|
|
Table 2. Physico-chemical barriers to infections
|
|
System/Organ |
Active component |
Effector Mechanism |
|
Skin |
Squamous cells; Sweat |
Desquamation; flushing, organic acids |
|
GI tract |
Columnar cells |
Peristalsis, low pH, bile acid, flushing, thiocyanate |
|
Lung |
Tracheal cilia |
Mucocialiary elevator, surfactant |
|
Nasopharynx and eye |
Mucus, saliva, tears |
Flushing, lysozyme |
|
Circulation and lymphoid organs |
Phagocytic cells
NK cells and K-cell
LAK
|
Phagocytosis and intracellular killing
Direct and antibody dependent cytolysis
IL2-activated cytolysis
|
|
Serum |
Lactoferrin and Transferrin |
Iron binding |
|
Interferons |
Antiviral proteins |
| TNF-alpha |
antiviral, phagocyte activation |
|
Lysozyme |
Peptidoglycan hydrolysis |
|
Fibronectin |
Opsonization and phagocytosis |
|
Complement |
Opsonization, enhanced phagocytosis, inflammation |
|
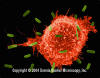 Figure 5
Figure 5
Macrophage Attacking E.coli (SEM x8,800) © Dr
Dennis Kunkel (used with permission)
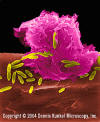 Figure 6
Figure 6
Alveolar (Lung) Macrophage Attacking E. coli
(SEM x10,000) ©
Dr Dennis Kunkel (used with
permission)
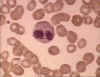 Figure 6A Eosinophil in blood film
© Bristol Biomedical Image Archive
Used with permission
Figure 6A Eosinophil in blood film
© Bristol Biomedical Image Archive
Used with permission
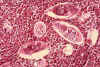 Figure 6B
Figure 6B
Histopathology of bladder shows eggs of Schistosoma haematobium surrounded by intense infiltrates of
eosinophils CDC/Dr. Edwin P. Ewing, Jr. epe1@cdc.gov
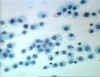 Figure 7
Figure 7
Histiocytes - Long lived resident macrophage found within tissues.
© Bristol Biomedical Image Archive
Used with permission
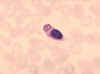 Figure 8 Monocyte with ingested malaria parasite.
CDC/Dr. Melvin
Figure 8 Monocyte with ingested malaria parasite.
CDC/Dr. Melvin
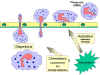 Figure
9
Chemotactic response to inflammatory stimulus Figure
9
Chemotactic response to inflammatory stimulus |
Cellular barriers to infection
Part of the inflammatory response is the recruitment of polymorphonuclear
eosinophiles and
macrophages to sites of infection. These cells are the main line of defense in
the non-specific immune system.
Neutrophils
Polymorphonuclear cells (PMNs, figure 4) are recruited to the
site of infection where they phagocytose invading organisms and kill them
intracellularly. In addition, PMNs contribute to collateral tissue damage that
occurs during inflammation.
Macrophages
Tissue macrophages (figure 5, 6, 7) and newly recruited monocytes (figure 4 and 8), which differentiate into macrophages, also function
in phagocytosis and intracellular killing of microorganisms. In addition,
macrophages are capable of extracellular killing of infected or altered self
target cells. Furthermore, macrophages contribute to tissue repair and act as
antigen-presenting cells, which are required for the induction of specific
immune responses.
Natural killer (NK) and lymphokine activated killer (LAK) cells
NK and LAK
cells can nonspecifically kill virus infected and tumor cells. These cells are
not part of the inflammatory response but they are important in nonspecific
immunity to viral infections and tumor surveillance.
Eosinophils
Eosinophils (figure 6a and b) have proteins in granules that
are effective in killing certain parasites.
PHAGOCYTOSIS AND INTRACELLULAR KILLING
Phagocytic cells
Neutrophiles/Polymorphonuclear cells
PMNs are motile phagocytic cells that have lobed nuclei. They can be identified
by their characteristic nucleus or by an antigen present on the cell surface
called CD66. They contain two kinds of granules the contents of which are
involved in the antimicrobial properties of these cells. The primary or
azurophilic
granules, which are abundant in young newly formed PMNs, contain cationic
proteins and
defensins that can kill bacteria, proteolytic enzymes like elastase, and
cathepsin G to breakdown proteins, lysozyme to break down bacterial cell walls,
and characteristically, myeloperoxidase, which is involved in the generation of
bacteriocidal compounds. The second type of granule found in more mature PMNs is
the secondary or specific granule. These contain lysozyme, NADPH oxidase
components, which are involved in the generation of toxic oxygen products, and
characteristically lactoferrin, an iron chelating protein and B12-binding
protein.
Monocytes/Macrophages
Macrophages are phagocytic cells that have a
characteristic kidney-shaped nucleus. They can be identified morphologically or
by the presence of the CD14 cell surface marker. Unlike PMNs they do not contain
granules but they have numerous lysosomes which have contents similar to the PNM
granules.
Response of phagocytes to infection
Circulating PMNs and monocytes respond to danger (SOS) signals generated at the site of an
infection. SOS signals include
N-formyl-methionine
containing peptides released by bacteria, clotting
system peptides, complement products and cytokines released from tissue
macrophages that have encountered bacteria in tissue. Some of the SOS signals
stimulate endothelial cells near the site of the infection to express cell
adhesion molecules such as ICAM-1 and selectins which bind to components on the
surface of phagocytic cells and cause the phagocytes to adhere to the
endothelium. Vasodilators produced at the site of infection cause the junctions
between endothelial cells to loosen and the phagocytes then cross the
endothelial barrier by “squeezing” between the endothelial cells in a process
called
diapedesis (Figure 9). Once in the tissue spaces some of the SOS signals
attract phagocytes to the infection site by chemotaxis (movement toward an
increasing chemical gradient). The SOS signals also activate the phagocytes,
which results in increased phagocytosis and intracellular killing of the
invading organisms.
|
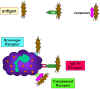 Figure 10
Adherence of bacteria via receptors
Figure 10
Adherence of bacteria via receptors |
Initiation of Phagocytosis (Figure 10)
Phagocytic cells have a variety of receptors on their cell membranes through
which infectious agents bind to the cells. These include:
Fc receptors
Bacteria with IgG antibody on their surface have
the Fc region exposed and this part of the Ig molecule can bind to the
receptor on phagocytes. Binding to the Fc receptor requires prior
interaction of the antibody with an antigen. Binding of IgG-coated
bacteria to Fc receptors results in enhanced phagocytosis and activation
of the metabolic activity of phagocytes (respiratory burst).
Complement receptors
Phagocytic cells have a receptor for the 3rd
component of complement, C3b. Binding of C3b-coated bacteria to this
receptor also results in enhanced phagocytosis and stimulation of the
respiratory burst.
Scavenger receptors
Scavenger receptors bind a wide variety of polyanions on bacterial surfaces resulting in phagocytosis of bacteria.
Toll-like receptors
Phagocytes have a variety of Toll-like
receptors (Pattern Recognition Receptors or PRRs) which recognize broad
molecular patterns called PAMPs (pathogen associated molecular patterns)
on infectious agents. Binding of infectious agents via Toll-like
receptors results in phagocytosis and the release of inflammatory
cytokines (IL-1, TNF-alpha and IL-6) by the phagocytes.
|
| |
Phagocytosis
After attachment of a bacterium, the phagocyte begins to extend
pseudopods around the bacterium. The pseudopods eventually surround the
bacterium and engulf it, and the bacterium is enclosed in a
phagosome.
During phagocytosis the granules or lysosomes of the phagocyte fuse with the
phagosome and empty their contents. The result is a bacterium engulfed in a
phagolysosome
which contains the contents of the granules or lysosomes.
|
|
|
|
|
|
|
 Figure11 Figure11
A. Respiratory burst: Oxygen-dependent,
myeloperoxidase-independent
reactions
 B. Respiratory burst: Oxygen-dependent,
myeloperoxidase-dependent reactions
B. Respiratory burst: Oxygen-dependent,
myeloperoxidase-dependent reactions |
Respiratory burst and intracellular killing
During phagocytosis there is an increase in glucose and oxygen consumption which
is referred to as the respiratory burst. The consequence of the respiratory
burst is that a number of oxygen-containing compounds are produced which kill
the bacteria being phagocytosed. This is referred to as oxygen-dependent
intracellular killing. In addition, bacteria can be killed by pre-formed
substances released from granules or lysosomes when they fuse with the phagosome.
This is referred to as oxygen-independent intracellular killing.
Oxygen-dependent myeloperoxidase-independent
intracellular killing (Figure11A)
During phagocytosis glucose is metabolized via the
pentose monophosphate shunt
and NADPH is formed. Cytochrome B which was
part of the specific granule combines with the plasma membrane NADPH oxidase
and activates it. The activated NADPH oxidase uses oxygen to oxidize the
NADPH. The result is the production of superoxide anion. Some of the
superoxide anion is converted to H2O2 and singlet oxygen by superoxide
dismutase. In addition, superoxide anion can react with H2O2 resulting in
the formation of hydroxyl radical and more singlet oxygen. The result of all
of these reactions is the production of the toxic oxygen compounds
superoxide anion (O2-), H2O2, singlet
oxygen (1O2) and hydroxyl radical (OH•).
Oxygen-dependent myeloperoxidase-dependent intracellular killing
(Figure
11B)
As the azurophilic granules fuse with the phagosome,
myeloperoxidase is released into the phagolysosome. Myeloperoxidase utilizes
H2O2 and halide ions (usually Cl-) to produce
hypochlorite, a highly toxic substance. Some of the hypochlorite can
spontaneously break down to yield singlet oxygen. The result of these
reactions is the production of toxic hypochlorite (OCl-) and singlet oxygen
(1O2).
Detoxification reactions (Table 3)
PMNs and macrophages have means to protect themselves from the toxic oxygen
intermediates. These reactions involve the
dismutation of superoxide anion to hydrogen peroxide by superoxide
dismutase and the conversion of hydrogen peroxide to water by catalase.
|
Table 3 |
|
Reaction |
Enzyme |
| H2O2 + Cl-
--> OCl- + H2O |
Myeloperoxidase |
| OCl- + H2O
--> 1O2
+Cl- + H2O |
| 2O2 + 2H+
--> O2-
+ H2O2 |
Superoxide dismutatse |
| H2O2 --> H2O + O2 |
Catalase |
Oxygen-independent intracellular killing (table 4)
In addition to the oxygen-dependent mechanisms of killing there are also
oxygen–independent killing mechanisms in phagocytes: cationic proteins (cathepsin)
released into the phagolysosome can damage bacterial membranes; lysozyme
breaks down bacterial cell walls; lactoferrin
chelates
iron, which deprives bacteria of this required nutrient; hydrolytic enzymes
break down bacterial proteins. Thus, even patients who have defects in the
oxygen-dependent killing pathways are able to kill bacteria. However, since
the oxygen-dependent mechanisms are much more efficient in killing, patients
with defects in these pathways are more susceptible and get more serious
infections.
|
Table 4. Oxygen-independent mechanisms of intracellular killing
|
|
Effector Molecule |
Function |
|
Cationic proteins (including cathepsin)
Lysozyme
Lactoferrin
Proteolytic and hydrolytic enzymes
|
Damage to microbial membranes
Splits mucopeptide in bacterial cell wall
Deprives proliferating bacteria of iron
Digestion of killed organisms |
|
|
 Figure
12
Nitric oxide-dependent killing Figure
12
Nitric oxide-dependent killing |
NITRIC OXIDE-DEPENDENT KILLING
Binding of bacteria to macrophages, particularly binding via Toll-like
receptors, results in the production of TNF-alpha, which acts in an
autocrine manner to induce the expression of the inducible nitric oxide
synthetase gene (i-nos ) resulting in the production of nitric oxide
(NO) (figure 12). If the cell is also exposed to interferon gamma (IFN-gamma)
additional nitric oxide will be produced (figure 12). Nitric oxide
released by the cell is toxic and can kill microorganism in the vicinity
of the macrophage.
|
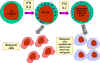 Figure 13 NK cells and their activation
Figure 13 NK cells and their activation
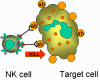 Figure 14
Figure 14
Killing of opsonised target by by K cell |
NON-SPECIFIC KILLER CELLS
Several different cells including NK and LAK cells, K cells, activated
macrophages and eosinophils are capable of killing foreign and altered self
target cells in a non-specific manner. These cells play an important role in the
innate immune system.
NK and LAK cells
Natural killer (NK) cells are also known as large granular lymphocytes (LGL)
because they resemble lymphocytes in their morphology, except that they are
slightly larger and have numerous granules. NK cells can be identified by the
presence of CD56 and CD16 and a lack of CD3 cell surface markers. NK cells are
capable of killing virus-infected and malignant target cells but they are
relatively inefficient in doing so. However, upon exposure to IL-2 and IFN-gamma,
NK cells become lymphokine-activated killer (LAK) cells, which are capable of
killing malignant cells. Continued exposure to IL-2 and IFN-gamma enables the
LAK cells to kill transformed as well as malignant cells. LAK cell therapy is
one approach for the treatment of malignancies.
How do NK and LAK cells distinguish a normal cell from a virus-infected or
malignant cell? NK and LAK cells have two kinds of receptors on their surface –
a killer activating receptor (KAR) and a killer inhibiting receptor (KIR). When
the KAR encounters its ligand, a killer activating ligand (KAL) on the target
cell the NK or LAK cells are capable of killing the target. However, if the KIR
also binds to its ligand then killing is inhibited even if KAR binds to KAL. The
ligands for KIR are MHC-class I molecules. Thus, if a target cell expresses
class I MHC molecules it will not be killed by NK or LAK cells even if the
target also has a KAL which could bind to KAR. Normal cells constitutively
express MHC class I molecules on their surface, however, virus infected and
malignant cells down regulate expression of class I MHC. Thus, NK and LAK cells
selectively kill virus-infected and malignant cells while sparing normal cells.
K cells (Figure 14)
Killer (K) cells are not a morphologically distinct type of cell. Rather a K
cell is any cell that mediates antibody-dependent cellular cytotoxicity (ADCC).
In ADCC antibody acts as a link to bring the K cell and the target cell together
to allow killing to occur. K cells have on their surface an Fc receptor for
antibody and thus they can recognize, bind and kill target cells coated with
antibody. Killer cells which have Fc receptors include NK, LAK, and macrophages
which have an Fc receptor for IgG antibodies and eosinophils which have an Fc
receptor for IgE antibodies.
|
| |
All components of the non-specific immune system are modulated
by products of the specific immune system, such as interleukins, interferon-gamma,
antibody, etc. |
|
At this time you should know the following:
1. Differences between the non-specific and specific
immune functions
2. Humoral components of the non-specific immune system
and their action
3. Cellular components of the non-specific immune function
and their action
4. Pathways of intracellular killing of bacteria by
phagocytes and their characteristic features
5. Effect of humoral components such as interferon,
TNF,
IL-2, complement etc. on cellular components of the non-specific immune
system
|
|
Table 5. Characteristics of cells involved in non-specific
resistance
|
|
Effector cell |
Identifying
marker(s) and/or function |
|
CD3 |
Ig |
Fc |
CD |
Phagocytosis |
|
Neutrophil
Macrophage
NK cell
K-cells
LAK cell
Eosinophil |
-
-
-
-
-
- |
-
-
-
-
-
- |
IgG
IgG
IgG
IgG
?
IgE |
CD67
CD14
CD56 & 16
?
?
CD67 |
+
+
-
-
?
-
|
|
|
 |
 Return to the Immunology Section of Microbiology and Immunology On-line
Return to the Immunology Section of Microbiology and Immunology On-line
This page last changed on
Saturday, December 09, 2017
Page maintained by
Richard Hunt
Please report any problems to richard.hunt@uscmed.sc.edu
|

 Figure 4A
Two neutrophils in blood film © Bristol Biomedical Image Archive
Used with permission
Figure 4A
Two neutrophils in blood film © Bristol Biomedical Image Archive
Used with permission
 Figure 5
Figure 5 Figure 10
Adherence of bacteria via receptors
Figure 10
Adherence of bacteria via receptors Figure 13 NK cells and their activation
Figure 13 NK cells and their activation













