|
KEYWORDS
Diplococcus
Pneumococcus
Autolysin
Bile solubility test
Optochin susceptibility
Capsule
Quellung reaction
Staphylococcus aureus
Staphylococcus epidermidis
Coagulase positive or Coagulase negative
Alpha,
beta, gamma and delta
cytotoxins
Leucocidin
Lipase
Exfoliatin
Enterotoxins
Toxic shock syndrome
Toxic shock toxin
Protein A
 Figure 1a Staphylococcus aureus - MRSA resistant coccoid prokaryote (dividing); causes food poisoning, toxic shock syndrome and skin and wound infections (scalded skin syndrome, scarlet fever, erysipelas, impetigo, etc.)
©
Dennis Kunkel Microscopy, Inc.
Used with permission
Figure 1a Staphylococcus aureus - MRSA resistant coccoid prokaryote (dividing); causes food poisoning, toxic shock syndrome and skin and wound infections (scalded skin syndrome, scarlet fever, erysipelas, impetigo, etc.)
©
Dennis Kunkel Microscopy, Inc.
Used with permission
 Figure 1b Staphylococcus aureus (Gram-positive)
© Copyright
Dr Linda M
Stannard,
1996. Used with permission
Figure 1b Staphylococcus aureus (Gram-positive)
© Copyright
Dr Linda M
Stannard,
1996. Used with permission
 Figure 1c Staphyllococcus aureus - Acridine-orange leucocyte cytospin test
© Bristol Biomedical Image Archive. Used with permission
Figure 1c Staphyllococcus aureus - Acridine-orange leucocyte cytospin test
© Bristol Biomedical Image Archive. Used with permission
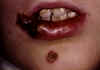 Figure 2 Staphylococcal Infection: Impetigo ©
Bristol Biomedical Image Archive. Used with permission
Figure 2 Staphylococcal Infection: Impetigo ©
Bristol Biomedical Image Archive. Used with permission
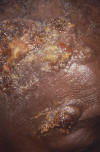 Figure 3
Figure 3
Impetigo lesions on forehead caused by Staphylococcus aureus
bacteria. CDC
 Figure 4
Figure 4
Box of Rely tampons. Associated with outbreak of toxic shock syndrome. CDC
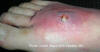 Figure 5b
Figure 5b
A cutaneous abscess on the foot caused by methicillin-resistant
Staphylococcus aureus. CDC
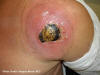 Figure 5c
Figure 5c
Cutaneous abscess caused by MRSA. CDC
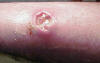 Figure 5d
Figure 5d
Cutaneous abscess caused by MRSA. CDC
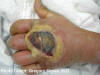 Figure 5e
Figure 5e
Cutaneous abscess caused by MRSA. CDC  Figure 6
Figure 6
S. epidermidis, the most common cause of blood stream infections
in patients with IVCs © Nancy
Khardori and Mahmoud Yassien, Southern Illinois University School of
Medicine, Springfield, Illinois and The
MicrobeLibrary
|
Staphylococci are facultative anaerobes. They
are Gram positive, occur in grape
like-clusters and are
catalase positive.
They are major components of the normal flora of
skin and nose in all people.
Staphylococcus aureus
Staphylococcus aureus (figure
1) is one of the commoner causes of opportunistic
nosocomial and community infections. These infections include pneumonia,
osteomyelitis, septic
arthritis, bacteremia,
endocarditis, abscesses/boils and other skin
infections (figure 2 and 3). S. aureus has gained notoriety
because of the increased incidence of Methicillin-resistant
Staphylococcus aureus (MRSA) Infections.
Pathogenesis
Food poisoning
S. aureus produces a number of toxins, of which the
enterotoxins (A, B, C and D) cause food poisoning. About a third to
a half of S. aureus strains produce enterotoxins which
are heat stable and thus survive cooking (boiling for 30minutes).
They are also resistant to proteolysis by intestinal proteases.
Food becomes contaminated with the
organism from human contact, grows and produces
enterotoxin. The organism does
not "infect" the patient on ingestion of contaminated food; rather the
pre-existing toxin causes the symptoms which include:
- vomiting
- nausea
- diarrhea
(watery and non-bloody, leading to dehydration)
- abdominal pain
Fever is not observed.
Because only the toxin is involved, onset of
symptoms occurs within a few hours and recovery occurs within a day.
Antibiotic treatment is not indicated because the bacteria are not
directly involved in causing the symptoms (and may, anyway, have
been killed by cooking).
Enterotoxins are
superantigens
that lead to cytokine production,
T cell activation, neutrophil infiltration with loss of small
intestine brush border cells. The release of inflammatory mediators
may be the cause of the characteristic S. aureus food
poisoning-associated vomiting.
Enterocolitis
The symptoms of enterocolitis are somewhat similar to food
poisoning (watery diarrhea and abdominal pain) but also include
fever. They are also produced by enterotoxin A and leukotoxin. The
cause is the treatment of patients with broad spectrum antibiotics
that allow S. aureus (which infects almost everyone) to grow
in the intestine in preference to the normal bacterial flora. The
bacteria can be detected in fecal samples.
Toxic shock syndrome
Toxic shock syndrome is caused by infection with
strains of S. aureus that produces toxic shock syndrome
toxin. It may be associated with a wound in which the bacteria
multiply rapidly but became particularly prominent to the public in the 1980's
when S.aureus infection was found to cause the toxic shock syndrome
that was seen after the use of certain tampons such as "Rely" (figure
4).
The bacteria were able to divide rapidly within the tampon; they do
not disseminate but remain in the vagina. However, the toxin does
disseminate and is responsible for the clinical features. This syndrome
includes:
Toxic shock syndrome toxin has the properties of a
superantigen, resulting in the production of
cytokines, vascular leak and cell toxicity. This results in
hypervolemic shock and death as a result of multi-organ failure.
Before the cause of toxic shock syndrome was discovered, the
mortality rate was high but now is around 5%. There can be recurrent
disease if the patient is not treated with the appropriate
antibiotic.
Toxic shock syndrome toxin
is involved in most menstruation-associated toxic shock syndrome. Enterotoxin B
is involved in many non-menstruation associated cases of toxic shock
syndrome.
Scalded skin
syndrome (Ritter disease, pemphigus neonatorum) and bullous impetigo
A minority of S. aureus
strains produce exfoliative toxins (A and B) and either toxin can cause scalded skin syndrome
or bullous impetigo in babies
and young children but rarely in adults. These toxins are serine
proteases that can digest, among other proteins, some of the
proteins found in
desmosomes, the structures that link epithelial cells together.
For example, the desmosomal protein called
desmoglein is digested between the cells of the
stratum granulosum epidermis. The process often resolves as the result
of the formation of protective neutralizing antibodies. Exfoliative toxins
are also superantigens.
Bullous impetigo is a mild form of S. aureus disease that usually occurs in newborn
infants and young children. It is manifested by large, flaccid
bullae
and attributed to S. aureus strains belonging to phage group
II capable of producing exfoliative toxins A and B
that separate the
stratum corneum
from the rest of the epidermis. The more common and milder form of
the disease (representing about 10% of all cases of
impetigo) differs from non-bullous impetigo in that the vesicles enlarge into
flaccid bullae before rupturing . The exposed skin surface is at first moist and
red, resembling a small burn. A thin, light-brown, “varnish-like” crust then
develops. Unlike the situation with scalded skin syndrome, bacteria can be
cultured from the fluid of the bullae and
Nikolsky's sign is absent.
The more severe form of disease with greater skin involvement
caused by the same staphylococcal strains is known as the
staphylococcal scalded skin syndrome. This also usually affects younger children.
The disease starts with local peri-oral erythema that spreads over
the whole body and progresses to widespread, flaccid bullae that rupture
causing exfoliation of the skin that resembles an extensive third-degree burn.
There are no organisms that can be cultured from the fluid of the
bullae, indicating that the bullae are caused by the toxin and not
the bacteria themselves. Before the bullae form, slight pressure on
the apparently normal epidermis may separate it at the basal layer.
It may be rubbed off when pressed with a sliding motion. This is
Nikolsky's sign.
This form of the disease can occur in epidemic form in nurseries, where it is
known as pemphigus neonatorum or Ritter’s disease. Fever and other systemic
symptoms are usually absent in the more localized forms of the
disease but are invariably present in patients with the
staphylococcal scalded skin syndrome.
Localized bullous impetigo is self-limited due to the formation
of neutralizing anti-toxin antibodies, and this is usually also the
case with staphylococcal scalded skin syndrome. However, the latter carries a significant mortality
rate (5%) that results from secondary bacterial infections of the areas where
the skin surface has been lost. Staphylococcal scalded skin syndrome
in adults is rare, and is usually associated with immunosuppression
or kidney disease. In this case mortality can be as high as half of
the patients. Cytotoxins
As noted above, S. aureus causes a number of different
disease entities associated with production of certain exotoxins. In addition to
these "disease-specific" exotoxins, other cell lytic exotoxins (alpha,
beta, gamma and delta toxins and leucocidins) may be
produced. These are also called cytotoxins because they cause cytolysis as a
result of plasma membrane damage. This leads to tissue destruction
as a result of lysosomal enzyme release.
Alpha toxin
This singler polypeptide toxin interacts
directly with the plasma membrane of many cells, embedding
itself in the lipid bilayer and forming pores that allow ions to
pass into and out of the cell. In particular, potassium ions are
lost and sodium and calcium enter the cell. This leads to
osmoticlysis. Alpha toxin is made by most S. aureus
strains.
Beta toxin
Beta toxin also damages cell membranes by
degrading specific lipids, sphingomyelin and lysophosptidyl
choline. The toxin is a sphingomyelase C and is also a single
polypeptide that is made by most S. aureus strains. It
appears that the degree of toxicity depends on the
concentrations of these lipids in the cell, both of which are
found primarily in the outer monolayer of the plasma membrane
bilayer.
Gamma toxins and P-V
leukocidin
Gamma toxins and Panton-Valentine leukocidin
consist of two polypeptide chains, an S chain and an F chain,
which together form pores in the plasma membranes of susceptible
cells. So far, three S chains and two F chains have been found
which can combine to form a number of different toxins that are
cytolytic to neutrophils and macrophages. The gamma toxins are
also hemolytic whereas P-V leukocidin is not. The gamma toxins
are also made by most S. aureus strains whereas P-V
leukocidin is made by only a minority of strains. It has been
particularly associated with virulent Methicillin-resistant
Staphylococcus aureus (MRSA) infections.
Delta toxin
This is a small protein that is cytotoxic to
many cells. It may act like a detergent, damaging cell membrane
bilayers resulting in cytolysis.
Other diseases
caused by S. aureus
Respiratory disease
Aspiration pneumonia can result from entry of
oral secretions into the lungs. The bacteria can cause local
abscesses and infiltrates. The disease is found in the very
young, the very old and patients with pulmonary disease. There
can also be spread of blood-borne organisms to the lungs,
causing hematogenous pneumonia. People with MRSA can get
necrotizing pneumonia which has a very high fatality rate.
Empyemia is an accumulation of pus in a cavity
of the body such as the lungs and is sometimes seen in pneumonia
patients. Many of these cases are the result of S. aureus
infections.
Bacteremia
S. aureus is found on the skin of most
people and can enter the body in wounds; however, many cases are
nosocomial and result from surgery or catheter use. The bacteria
may disseminate throughout the body.
Endocarditis
Endocarditis is an inflammation of the
endocardium (the inner layer of the heart) and usually involves
the heart valves (native or prosthetic valves). S. aureus-associated
endocarditis can have a high mortality rate.
Urinary tract
infections
Complicated urinary tract infections occur in specific clinical settings. Renal
abscess can result from hematogenous seeding of the renal cortex (most often due
to S. aureus) or from ascending infection leading to severe pyelonephritis (most
often due to gram-negative rods).
Dissemination to other
parts of the body
S. aureus bacteremia can disseminate via
the bloodstream to other parts of the body causing disease. Such
sites include bone giving rise to S. aureus osteomyelitis
resulting in pain, fever and sometimes a
Brodie abscess and
septic arthitis.
Skin disease
Folliculitis
Folliculitis, by which is meant
pyoderma involving the hair follicles and
apocrine glands, affects nearly everyone at one time or
another but is usually self-limited. Occasionally,
folliculitis evolves into larger lesions known as
furuncles and
carbuncles.
S. aureus is the usual cause of folliculitis in non-immunocompromised
patients, the infection probably arising from prior nasal
colonization by this bacterium.
Furuncles, carbuncles and skin abscesses
The familiar furuncle or “boil” is thought
to arise from folliculitis. The term furunculosis refers to
multiple boils or to frequent recurrences. Carbuncles are
more extensive and difficult-to-treat lesions that often
require surgical intervention. Skin abscesses, although
similar to carbuncles histologically, are usually deeper
infections that do not originate in hair follicles.
S. aureus is the usual cause of both
furuncles and carbuncles, and is also the sole or
predominant pathogen in about 50% of skin abscesses.
Predisposing factors to recurrent furuncles (furunculosis)
include obesity, corticosteroid therapy, disorders of
neutrophil function, and possibly diabetes mellitus.
Immunoglobulin levels are usually normal in patients with
furunculosis (low IgM levels have been demonstrated in some
patients but this is of uncertain significance and, in
contrast to IgG deficiency, replacement therapy is
impractical). Most patients with recurrent furuncles have no
obvious predisposing factors other than being nasal carriers
of S. aureus nasal carriers. Outbreaks of
furunculosis have been described in families, athletic
teams, and in village residents who took steam baths
together. Skin abscesses can result from minor trauma,
injecting drug use (the practice of subcutaneous and
intramuscular injection is known as “skin popping”), or
bacteremia. Congenital immunodeficiency syndromes such as
the hyperimmunoglobulin E-recurrent infection syndrome
(Job’s syndrome) are sometimes present in patients with
recurrent skin abscesses. Rarely, skin abscesses are
self-inflicted (factitious abscess), in which case Gram’s
stain and culture may reveal “mouth flora” bacteria.
For more information see:
Infectious disease - skin and bone
Other secreted
enzymes
S. aureus strains
secrete a number of tissue-degrading enzymes that may result in
tissue damage. These include
lipases, nucleases, hyaluronidase,
coagulase and
plasmin.
One form of coagulase is bound to the S. aureus surface and
converts fibrinogen to fibrin. This insoluble protein causes the
bacteria to aggregate. The other coagulase is secreted and combines
with coagulase-reacting factor in the serum resulting in the
formation of staphylothrombin that, like normal thrombin, also forms
insoluble fibrin. This may also be anti-phagocytic.
Protection
against phagocytosis
In addition, to the toxins and enzymes that directly
damage cells and tissues described above, S. aureus strains
produce other proteins involved in pathogenesis. For example, these
bacteria have two mechanisms that protect them against phagocytosis
by polymorphonuclear leukocytes and other phagocytic cells.
-
Although the bacteria are
opsonised by proteins in serum, the capsule and slime layer
protect the cells against phagocytosis.
-
Protein A is found on the surfaces of most S.
aureus strains. It binds to immunoglobulin
G and complement, blocking Fc and complement receptors and is thus anti-phagocytic.
Identification
-
S. aureus
is beta-hemolytic on sheep blood agar
-
Ferments mannitol (figure 9)
-
Is often golden pigmented (hence
the name aureus)
-
Is coagulase-positive
-
Presence of protein A
In reference laboratories phage-typing is used.
Methicillin-resistant
Staphylococcus aureus (MRSA) Infections
Methicillin-resistant Staphylococcus aureus (MRSA)
is defined as any strain of Staphylococcus aureus that has
developed resistance to
beta-lactam antibiotics (such as
penicillins) and cephalosporins. This results from the
production of a phage-coded penicillinase that degrades beta
lactam antibiotics. Some strains also have modified penicillin binding proteins.
Many healthy people carry MRSA asymptomatically.
Patients with compromised immune systems are at a significantly greater
risk of symptomatic infections. Apparently healthy people may have
simple topical skin infections (noted above) but in some people MRSA may
progress rapidly within a day or two of initial topical symptoms. In
these patients, after about 72 hours, MRSA may invade tissues and
become resistant to treatment.
The majority of community-associated MRSA infections are
localized to skin and soft tissue and usually can be treated effectively
but some strains exhibit enhanced virulence and spread into the tissues,
causing illness much more severe than traditional nosocomial MRSA
infections.
At first, MRSA is characterized by small red pimples and
there may be fever and a rash. As the infection progresses over a period
of a few days, the pimples increase in size and become more painful.
Eventually, they form deep, pus-filled boils (figure 5b-e). The
infection can disseminate throughout the body (sepsis) and vital organs
may be affected. This can lead to toxic shock syndrome, and necrotizing
pneumonia, some times referred to as "flesh eating" pneumonia. In
hospitals, there can be surgical site infections.
Epidemiology
Two per cent of people carry MRSA. Currently, in the
United States, there are about 75,000 cases of invasive MRSA
Infections per year, of which about 14,000 are in dialysis patients.
Nosocomial invasive MRSA infections declined 54% between 2005 and
2011, with 30,800 fewer severe MRSA infections. In addition, there
were 9,000 fewer deaths in hospital patients in 2011 versus 2005.
MRSA is usually spread by direct contact with an
infected wound or from contaminated hands, usually those of
healthcare providers. People who carry MRSA but do not have signs of
infection can spread the bacteria to others and potentially cause an
infection.
Diagnosis
This can be done by growth of the organism in the
laboratory. There are more rapid tests available such as
quantitative PCR.
Treatment
Intravenous
vancomycin and teicoplanin are used
to treat MRSA but some new MRSA strains are resistant to these
antiboitcs also.
Daptomycin is often used to treat these strains
Staphylococcus epidermidis
Staphylococcus epidermidis (figure 6) is a major component of the
normal skin flora and thus commonly a contaminant of cultures in laboratories.
It
is a less common cause of opportunistic infections than
S. aureus,
but is still significant. Normally, infections are nosocomial. The bacteria form
biofilms on catheters, shunts, artificial heart valves and other surgical
devices and can cause endocarditis and sepsis.
The formation of biofilms is import in the virulence of
the bacteria. It is likely that the bacteria bind blood proteins and
extracellular matrix proteins to their surface. The bacteria also
produce a sulfated polysaccharide extracellular coat called
polysaccharide intercellular adhesion. Other bacteria bind to this
surface coat making a multilayer biofilm. The cells within the biofilm
become partially metabolically inactive and this, together with the
difficulty in penetrating the biofilm with antibiotics, makes it
difficult to treat the infection. In addition, S. epidermidis
strains are often antibiotic-resistant (including penicillin,
amoxicillin, and methicillin).
Since antibiotics are largely ineffective in clearing
biofilms, the usual tratment is to chnage the infected medical device.
The drug of choice is usually vancomycin, to which rifampin or
aminoglycoside can be added.
Identification
|

 Figure 7 Two different species of Staphylococcus
growing on mannitol salt agar (MSA). MSA is selective because it
contains 7.5% salt–a high salt concentration that promotes the growth
of some organisms while discouraging the growth of others. MSA is
a differential medium because it contains the sugar mannitol and the pH
indicator phenol red. Organisms that can ferment mannitol produce
acid by-products, causing a color change. Phenol red is a cherry
red color above pH 8.5, yellow-red from pH 6.9 to 8.5, and bright yellow
at pH 6.9 or lower. Although both Staphylococcus epidermidis and
Staphylococcus aureus can tolerate the high salt content of MSA,
only S. aureus can ferment mannitol, causing the phenol red in
the medium to turn yellow.
© Margaret (Peg) Johnson, Mesa Community College, Mesa, Arizona and
The MicrobeLibrary
Figure 7 Two different species of Staphylococcus
growing on mannitol salt agar (MSA). MSA is selective because it
contains 7.5% salt–a high salt concentration that promotes the growth
of some organisms while discouraging the growth of others. MSA is
a differential medium because it contains the sugar mannitol and the pH
indicator phenol red. Organisms that can ferment mannitol produce
acid by-products, causing a color change. Phenol red is a cherry
red color above pH 8.5, yellow-red from pH 6.9 to 8.5, and bright yellow
at pH 6.9 or lower. Although both Staphylococcus epidermidis and
Staphylococcus aureus can tolerate the high salt content of MSA,
only S. aureus can ferment mannitol, causing the phenol red in
the medium to turn yellow.
© Margaret (Peg) Johnson, Mesa Community College, Mesa, Arizona and
The MicrobeLibrary











