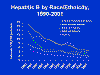More than 70% of patients infected with hepatitis C in the United States are infected with genotype 1. They require interferon plus ribavirin together with sofosbuvir over a period of 12 weeks. In a clinical trial, about 90 percent of previously untreated patients taking sofosbuvir in combination with interferon and ribavirin showed no detectable virus in the blood at the end of treatment.

CHAPTER NINETEEN - HEPATITISPART TWO - DISEASE TRANSMITTED PARENTERALLY
HEPATITIS B, C, D AND G
Dr Richard Hunt
Professor
Department of Pathology, Microbiology and Immunology
University of South Carolina School of Medicine
FEEDBACK
 Figure 1
Figure 1Hepatitis B virus CDC
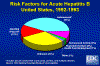 Figure 2
Figure 2
Risk Factors for Acute Hepatitis B, United States, 1992-1993 CDC
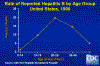 Figure 3
Figure 3
Rate of Reported Hepatitis B by Age Group, United States, 1990. CDC
Hepatitis B virus (HBV) (figure 1) belongs to the hepadnavirus family and has a DNA
genome that is replicated via an RNA intermediate. Depending on the
patient’s immune response, infection by HBV can be asymptomatic, chronic or
acute. As with HAV, humans are the only reservoir for HBV. The virus is
spread via contact with body fluids (usually blood contamination but the
virus is also in semen and various secretions such as vaginal fluids,
menstrual blood, saliva and milk). Although injection of blood (as a result
of intravenous drug use or the use of another blood contaminated instrument
such as a razor) is the most common route of infection, the virus can also
be contracted via sexual intercourse (particularly male to male) and
perinatally (figure 2). According to the Centers for Disease Control approximately
78,000 people in the United States were infected by HBV in 2001 and about
5,000 people die per year from HBV-associated disease. One in 20 people in
the United States is infected by HBV at some time in their lives with the
highest infection rate being in young adults (figure 3 and 4). About 5%
of people infected by HBV get a chronic infection and there are more than
one million Americans with chronic hepatitis B. Up to one quarter of these
chronically-infected patients will die of some form of liver disease. As a
result of the currently available excellent vaccine, the number of acute
hepatitis B infections in the United States has been falling (figure 5).
 Figure 4
Figure 4Age at Aquisition of Acute and Chronic HBV Infection
United States, 1989 Estimates CDC
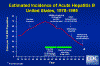 Figure 5
Figure 5
Estimated Incidence of Acute Hepatitis B, United States, 1978-1995 CDC
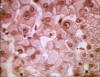 Figure 6
Figure 6
Australia antigen carrier, ground glass hepatocytes ©
Bristol Biomedical Image Archive. Used with permission
Pathology
HBV enters the body in the bloodstream and targets hepatocytes, presumably since its receptor is found predominantly on these cells. There is little cytopathic effect and the rate at which symptoms appear depends on the initial dose of virus. The incubation period is 60-90 days (range 45-180 days), although virus replication starts a few days after infection. The first sign of infection is the characteristic appearance of HBsAg in infected cells (ground glass appearance (figure 6)). As with other hepatitis viruses, the symptoms are immune-mediated, resulting from inflammation and cell-mediated (cytotoxic T cell) responses to HBsAg on the surface of hepatocytes. These also resolve the disease. Symptoms include jaundice (figure 7), fatigue, abdominal pain, loss of appetite, nausea, vomiting and joint pain. If the cell-mediated immune response is weak, symptoms are mild but the infection does not resolve and chronic hepatitis ensues. This is frequently the case with younger patients who have lesser cell-mediated immunity. About 10% of patients less than five years of age show clinical illness (as exemplified by jaundice) although 30-90% of infected young patients go on to chronic infection. In patients of five years or older, 30-50% have clinical illness and about one third are asymptomatic. In these patients, 2 to 10% go on to chronic infection (figure 8).
Chronic HBV infection can lead to chronic hepatitis. This leads to cirrhosis of the liver in up to a quarter of patients within five years. Of these patients, up to one quarter will develop hepatocellular carcinoma (figure 9) or liver failure. Both of these are fatal in the absence of a liver transplant.
 Figure 7
Figure 7Acute Viral Hepatitis CDC
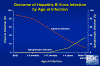 Figure 8
Figure 8Outcome of Hepatitis B Virus Infection by Age at Infection CDC
 Figure 9
Figure 9
Khmer woman who died of hepatoma, four months after arriving in a refugee
camp in Thailand CDC
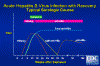 Figure 10
Figure 10Acute Hepatitis B Virus Infection with Recovery - Typical Serologic Course CDC
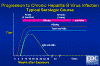 Figure 11
Figure 11
Progression to Chronic Hepatitis B Virus Infection - Typical Serologic
Course CDC
Immunology
A cell-mediated response to an HBV infection results in cytotoxic T cells against both surface (HBsAg) and internal antigens (HBcAg and HBeAg). The humoral response is also protective. In addition to intact virions, HBV-infected cells shed particles that are composed primarily or completely of aggregated HbS antigen. This combines with and blocks anti-HbS antibodies, thereby limiting the humoral response. The large amounts of antibody-HBsAg complexes cause type III hypersensitivity reactions and result in rash, arthralgia and damage to the kidneys. Nevertheless, HBsAg antibody confers life-long immunity and the presence of HBeAg indicates low transmissibility.
In an acute HBV infection, symptoms last from 10 to 20 weeks after infection. Before symptoms appear HBsAg and HBeAg are detectable in the bloodstream. Antibodies against HBeAg are detectable about 4 months after infection. Initially, anti-HBcAg antibodies are IgM but this wanes although total anti-HBcAg continues at a high level. HBsAg is detectable in the bloodstream from one to six months after infection, but anti-HBsAg is only detectable from about 8 months. Thus, there is a "window" in which neither HBsAg nor anti-HBsAg antibodies can be detected. As a result of the immune response, the disease resolves in most patients (figure 10).
In a chronic infection, HBsAg and HBeAg are detectable throughout the course of the infection. Anti-HBcAg (again initially IgM) and anti-HBeAg are also detectable (figure 11).
Carcinogenesis
HBV is a major cause of hepatocellular carcinoma worldwide (figure 9); in fact, HBV infection may be the cause of over 80% of primary hepatocellular carcinoma cases worldwide make account for. This is particularly the case when the patient is HBeAg-positive. In Taiwan where 15% of the population is carriers, HBsAg carriers have a risk of hepatocellular carcinoma that is 217 times that of a non-carrier. About half of deaths of HBsAg carriers are caused by liver cirrhosis or hepatocellular carcinoma compared to 2% of the general population. For more information on hepadnaviruses and cancer, see chapters six and eighteen.
 Figure 12
Figure 12Geographic distribution of chronic HBV infection CDC
Epidemiology
HBV is found worldwide (figure 12). The highest incidence of HBV seropositivity (anti-HBsAg) is in sub-Saharan Africa, the Far-East (China, Malaysia, Indonesia, Philippines, Papua New Guinea etc). Other high levels (more than 8% of the population infected) are in northern South America, northern Canada and Alaska and Greenland. In these areas, the lifetime risk of infection is more than 60% with infections in childhood especially common. In areas of low seropositivity (less than 2% of the population with anti-HBsAg antibodies), the lifetime risk of HBV infection is less than 20% with most infections occurring in adults who are in elevated risk groups. In the United States, HBsAg seropositivity occurs in less than 2% of the population but in Asian Americans chronic HBV may be as high as 10-15% of the population. In Asian Americans, hepatocellular carcinoma is a leading cause of death. Half of all children born to mothers with chronic HBV infection in America are Asian Americans. African Americans also show a high rate of HBV seropositivity (figure 13).
HBV is spread parenterally, sexually (hetero and homosexually) and neonatally. The virus is high in the blood/serum and in wounds. Moderate levels are found in semen, saliva and vaginal secretions. Other body secretions show low or non-detectable levels of virus.
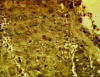 Figure 14 Immunohistochemistry - Hepatitis B antigen positive
© Bristol Biomedical Image Archive. Used with
permission
Figure 14 Immunohistochemistry - Hepatitis B antigen positive
© Bristol Biomedical Image Archive. Used with
permission
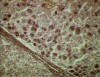 Figure 15
Figure 15
Cirrhosis and hepatitis B antigen positive
© Bristol Biomedical Image Archive. Used with
permission
Diagnosis
Serum hepatitis is usually first diagnosed from the clinical symptoms. Liver enzymes are also detected in the bloodstream during the symptomatic phase. Much further information can be obtained from serology and the presence of HBV antigens. Thus, an acute infection can be distinguished from a chronic infection by the presence of antibodies (IgM) against HBcAg. Tests that detect HBsAg and HBcAg and antibodies against HBcAg, HBsAg and HBeAg (the hepatitis B panel) are used in diagnosis. Because of the large amounts of HBsAg that are not associated with infectious virus, the presence of HBeAg is the best marker for infectious virus. As shown in figure 10, detectable anti-HBsAg antibodies do not rise until about eight months after infection while the antigen, HBsAg, is detectable much earlier and then subsides. The failure to detect anti-HBsAg early in infection is not because of a lack of the antibodies; instead, they are undetectable because they are complexed with the large amount of the antigen that is shed from infected cells. The period from about six to eight months when neither free HBsAg nor its antibody can be detected is known as the “HBsAg window”. This phenomenon also applies to HBeAg which is shed from infected cells, though to a much lesser extent; thus, the best tool for diagnosis of an acute HBV infection during the window is the presence of anti-HBc IgM. For more information see table 1.
HBV can also be detected in the laboratory by immunohistochemistry (figure 14-16).
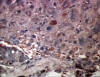 Figure 16
Figure 16Hepatocellular carcinoma and hepatitis B antigen positive © Bristol Biomedical Image Archive. Used with permission
| Table 1 - The HBV Panel - Interpretation | ||
| Test | Results |
Interpretation |
| HBsAg anti-HBcAg anti-HBsAg |
Negative Negative Negative |
The patient is
susceptible to an HBV infection and has not been exposed previously to
the virus The patient has not been vaccinated |
| HBsAg anti-HBcAg anti-HBsAg |
Negative Positive Positive |
The patient is immune to HBV as a result of having been infected previously (indicated by the presence of anti-HBc antibodies which would not occur if the patient had been vaccinated) |
| HBsAg anti-HBcAg anti-HBsAg |
Negative Negative Positive |
The patient is immune because of vaccination against HBV |
| HBsAg anti-HBcAg anti-HBcAg IgM anti-HBsAg |
Positive Positive Positive Negative |
The patient has an acute HBV infection. Any anti-HBsAg antibodies that have been made are complexed with the large amount of the antigen and are thus undetectable |
| HBsAg anti-HBcAg anti-HBcAg IgM anti-HBsAg |
Positive Positive Negative Negative |
The patient has a chronic HBV infection. The IgM anti-HBc has waned |
| HBsAg anti-HBcAg anti-HBsAg |
Negative Positive Negative |
The patient may be in
the recovery phase of an acute HBV infection. This patient could be
infected and thus a carrier of HBV. The inability to detect HBsAg may
result from it being complexed with anti-HBsAg antibodies in the
"window" phase Other possible interpretations are that the patient
is distantly immune to HBV but the test was too insensitive to detect
anti-HBsAg. There may also have been a false positive for anti-HBcAg
and the patient is actually uninfected. |
 Figure 17
Figure 17Recommended post-exposure prophylaxis for exposure to HBV CDC
Treatment
Supportive care is the major treatment. Anti-HBV immune globulin is effective soon after exposure (figure 17). It can also be given neonatally to children of HBsAg-positive mothers. Ideally, the immune globulin should be administered within 24 hours of birth or exposure and is probably not effective after one week from exposure.
There are three FDA-approved drugs for treating hepatitis B.
- Interferon-alpha 2b (Intron A - Schering-Plough) is a protein that mimics the cell’s natural defenses against viral infection.
- Hepsera (Adefovir Dipivoxil – Gilead Sciences) is a nucleotide analog that inhibits HBV DNA polymerase (reverse transcriptase). Use is indicated for the treatment of chronic hepatitis B in adults with evidence of active viral replication and either evidence of persistent elevations in serum aminotransferases (ALT or AST) or histologically active disease.
- Lamivudine (Epivir HBV - Glaxo SmithKlein). This is 3TC which is a reverse transcriptase inhibitor that is also approved for use inn HIV infections. As with all reverse transcriptase inhibitors, the appearance of resistant mutants is a problem. Hepsera can be used in patients with Epivir-resistant mutant virus.
Vaccination
This is the best preventative strategy. The current vaccines are subunit vaccines made in yeast that has been transfected with a plasmid that contains the S gene (that codes for HBsAg). The HBV vaccines go under the names of Recombivax-HB (Merke) and Energix-B (Glaxo). In addition, there is an approved vaccine against both HAV and HBV (Twinrix – Glaxo). Another formulation for infants (Pediarix – Glaxo) contains vaccines against diphtheria, tetanus, pertussis (whooping cough), polio and HBV.
For vaccination of infants, there are several options depending on whether the mother is HBsAg positive. In the latter case, the vaccine is given along with HBV immune globulin. If the mother is seronegative, the vaccine alone is given. There are normally three vaccinations for children (birth, 1 and 6 months) or adults to provide protective immunity. The vaccine is recommended for children up to 18 years and for adults at high risk.
 Figure 18
Figure 18Hepatitis Delta agent CDC
HEPATITIS D - DELTA AGENT
Hepatitis D (HDV) or delta agent (figure 18) is a defective virus with some similarities to plant viroids. It cannot code for its own surface protein and thus in order to produce more virus particles, it needs a helper virus; this is HBV. HDV is either acquired along with HBV (co-infection) or as a super-infection of an already HBV-infected individual.
Co-infection by HDV and HBV exacerbates the acute disease and fulminant hepatitis is more likely than with HBV alone. The likelihood of chronic HBV infection is, however, less in co-infected people. Super-infection leads to more rapid manifestation of the disease because co-infection requires HBV replication before HDV replication can occur. CDC studies show that HBV carriers super-infected with HDV have a 70-80% chance of chronic liver disease accompanied by cirrhosis compared with a rate of 15-30% in patients who are only infected by HBV.
Transmission
HDV is usually transmitted via similar means to HBV, that is by intravenous drug use and by sexual contact with the latter being less efficient. Perinatal infection is infrequent.
 Figure 19
Figure 19HBV - HDV Coinfection Typical Serologic Course CDC
 Figure 20
Figure 20
HBV - HDV Superinfection . Typical Serologic Course CDC
Immunology
In an HBV-HDV co-infection (figure 19), anti-HDV IgG and IgM are usually seen but HDV antigen is only detected in about a quarter of patients. HDV antigen, when it is seen, disappears when HBsAg appears. In a minority of patients, IgM is found early in acute disease or IgG is seen later. The rise in antibodies during the symptomatic phase declines as the symptoms resolve and, unlike anti-HBsAg, there are no antibodies to show that the patient was once HDV-infected. As with a simple HBV infection, anti-HBsAg rises after the level of the antigen has declined with a “window” period between the two.
In a super-infection by HDV (figure 20), anti-HBsAg levels fall as anti-HDV antigen rises. In this case the HDV antigen and the virus (as detected by the presence of its RNA) remain because super-infection usually leads to chronic HDV infection. In this chronic infection, IgG and IgM antibodies against HDV antigen persist.
Pathology
As with the other viral hepatitis disease, the symptoms are caused by the immune reaction of the patient rather than to direct cytotoxic effects of the virus infection of hepatocytes. There is more severe disease when HDV super-infects an already HBV-infected patient. In super-infection of chronically-infected HBV-infected patients, about half of the patients exhibit acute hepatitis that resolves. About 10-40% get chronic persistent hepatitis and 7-10% get fulminant hepatitis. In co-infection, most (90%) patients exhibit acute hepatitis that resolves and less than 10% get chronic hepatitis. Only 2-4% exhibit fulminant hepatitis. Thus, fulminant hepatitis is more common in HDV-infected patients than in patients infected with other hepatitis viruses.
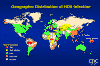 Figure 21
Figure 21Geographic Distribution of HDV Infection CDC
Epidemiology
HDV is found worldwide (figure 21) and since HBV infection is also necessary, distribution is much the same as HBV. When the incidence of chronic HBV infection is low, HDV incidence is also low and is mostly seen in intravenous drug users. HDV infection rate is variable in areas of moderate and high HBV infection. For example, in Russia, Rumania and southern Italy, HDV infection is very high in HBV-infected people. In other parts of Italy and northern Africa, HDV is moderately common while in south Asia with its extremely high HBV incidence, HDV incidence is low. In some South American countries there are isolated periodic epidemics of HDV in the HBV-infected population. These outbreaks can lead to very severe disease involving fulminant hepatitis with a 10-20% fatality rate.
Diagnosis
There are commercially available tests that detect anti-HDV IgG
Treatment and Prevention
Since HDV depends on HBV for a productive infection, HBV/HDV co-infection can be prevented by HBV prophylaxis using the very effective HBV vaccine. There is no prophylaxis for HDV super-infection which can be diminished by education of HBV-infected patients such as counseling against intravenous drug use.
 Figure 22
Figure 22
Estimated Incidence of Acute HCV Infection United States, 1960-2001 CDC
 Figure 23
Figure 23
Prevalence of HCV Infection by Age and Gender, United States, 1988-1994 CDC
NON-A, NON-B HEPATITIS (NANBH) - HEPATITIS C
Hepatitis C (HCV) is a flavivirus for which the only known reservoirs are humans and chimpanzees; in fact, the virus was first identified in chimpanzee blood. HCV causes “non-A, non-B hepatitis” and was the major cause of post-transfusion hepatitis before routine screening of the blood supply for HCV. Worldwide, there are approximately 200 million HCV carriers.
In the United States, there are about 2.4 to 3 million people who are chronically HCV infected and between 3.1 to 4.1 million people have had an HCV infection at some time in their life. There were 25,000 new HCV infections in 2001 (down from 242,000 in 1985-89 as a result of blood screening and a decline in intravenous drug users) (figure 22); this virus causes 40-60% of chronic liver disease leading to 10,000 to 12,000 deaths per year. HCV seropositivity is higher in males in the 30-39 years age group (figure 23).
Although HCV, like HBV, can cause chronic persistent hepatitis, deaths from acute liver failure are rare. HCV infection usually results from (figure 24-26):
- Use of intravenous drugs. This a highly efficient route of infection. Among needle-sharing drug users, there is a 30% prevalence of HCV infection within three years of initiation and greater than 50% after five years. Most (50-90%) HIV infected intravenous drug users are also HCV-infected.
- Blood transfusion or tissue transplantation. Since the introduction of blood screening, the incidence of transfusion-associated HCV infections has fallen (figure 27).
- Occupational exposure to blood and other fluids. This is rather inefficient with a 1.8% incidence of infection after a needle stick from an HCV-positive person. Splashes of blood or contact with a wound may also result in rare transmission. 1-2% of heath care workers in the United States are HCV-positive (which is lower than the general population)
- Inadvertent (iatrogenic/nosocomial) transmission as a result of inoculations
- An HCV-infected mother at the time of delivery. About 6% of babies born to infected mothers become HCV-infected but this rises to 17% if the mother is also HIV-infected. It does not appear that HCV can be transmitted by breastfeeding. Infected infants do well and severe HCV-hepatitis is rare.
- Sexual intercourse with an infected person. The risk is increased with multiple sex partners but the efficiency of spread is low and what influences transmission is unknown. Transmission from a male to a female is more efficient than male to male; however, sexually-transmitted HCV hepatitis constitutes about 20% of acute and chronic HCV infections in the United States.
 Figure 24
Figure 24Reported Cases of Acute Hepatitis C by Selected Risk Factors, United States, 1982-2001 CDC
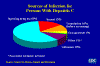 Figure 25
Figure 25 Sources of Infection for Persons With Hepatitis C CDEC
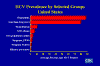 Figure 26
Figure 26
HCV Prevalence by Selected Groups United States CDC
 Figure 27
Figure 27Post-transfusion Hepatitis C CDC
Pathogenesis
HCV enters the bloodstream and infects hepatocytes. The virus usually does not kill the host cell and thus can set up a persistent infection leading to chronic disease. Symptoms, similar to HBV, again do not result from the virus but from the effect of the immune system on infected hepatocytes, the cytotoxic T cell response being the most important factor.
Viremia is detected one to three weeks after infection. After a prodromal phase of six to seven weeks (although ranging from two to twenty six weeks), symptoms appear (jaundice, abdominal pain, nausea, appetite-loss and dark urine). These are usually milder than with HBV and in more than 80% of patients the acute phase of viremia is asymptomatic. In the acute phase of infection, virus particles can be detected for several months. In 15-25% of patients, the virus is cleared ending the infection but, in the majority of infected people, HCV sets up a persistent liver infection that may last for many years leading to chronic active hepatitis. Many of these patients develop cirrhosis of the liver and some experience liver failure. During the chronic infection period, HCV can give rise to extra-hepatic manifestations; these include essential mixed cryoglobulinemia (resulting in rash, vasculitis etc), porphyia cutanea retarda, membranoproliferative glomerulonephritis and possibly diabetes mellitus and lymphoma. These symptoms may be due to immune complexes that are formed and also to autoimmune disease. Pathogenicity of HCV infections is promoted under a variety of conditions including increased consumption of alcohol and co-infection with HIV or chronic HBV. It is also greater in older people (more than 40 years) and in males.
Carcinogenesis
After many years (up to thirty), a small proportion (5%) of HCV chronically-infected patients develop hepatocellular carcinoma.
 Figure 28 Serologic pattern of acute HCV infection with recovery
Figure 28 Serologic pattern of acute HCV infection with recoveryCDC
 Figure 29 Serologic Pattern of Acute HCV Infection with Progression to
Chronic Infection CDC
Figure 29 Serologic Pattern of Acute HCV Infection with Progression to
Chronic Infection CDCImmunology
Symptoms, when they occur, extend from one to more than five months after infection; virus is detectable in the bloodstream during this period. Also during this period, liver enzymes, such as alanine aminotransferase, are elevated (figure 28). Anti-HCV antibodies rise after two months and are detectable for several years if the patient is chronically infected (figure 29). There are six serotypes of HCV that circulate worldwide with multiple subtypes.
Epidemiology
HCV is found worldwide with the highest incidence in southern and central Europe, the Middle East and Japan.
 Figure 30
Figure 30HCV Infection Testing Algorithm for Diagnosis of Asymptomatic Persons CDC
Diagnosis
Symptoms are the first aspect of diagnosis. These include jaundice, nausea and fatigue accompanied by elevated (at least ten fold) alanine aminotransferase. Antibodies against HCV are also clearly indicative. There is a highly specific ELIZA test that detects HCV antibodies; however, these do not appear until eight to twenty weeks after infection which is after the end of the prodromal phase. Thus, antibody is not a reliable indicator of acute infection. PCR can be used to detect viral RNA within a week or two of infection in seronegative patients. There is also a recombinant immunoblot assay that detects two or more HCV antibodies. If the patient has used intravenous drugs, received clotting factor made before 1987, had an organ transplant or blood transfusion before 1992, has been subjected to hemodialysis or has evidence of liver disease, HCV testing is recommended. People who have been occupationally exposed to HCV-infected blood or children of infected mothers should also be tested (figure 30).
Chronic infection can be diagnosed from the presence of antibodies and long term elevation of serum aminotransferases. This can be confirmed by PCR since in a chronic infection, viral RNA should be present in the bloodstream. In immuno-suppressed patients, PCR testing is necessary and this is also the case when other liver-damaging behavior, such as alcoholism, is suspected. Other problems that may be confused with HCV hepatitis are autoimmune hepatitis, chronic hepatitis B and D, alcoholic hepatitis, non-alcoholic steatohepatitis (fatty liver), sclerosing cholangitis, Wilson's disease, alpha-1-antitrypsin-deficiency-related liver disease and drug-induced liver disease (National Institute of Diabetes and Digestive and Kidney Diseases, National Institutes of Health).
Treatment
The patient should be assessed for chronic liver disease and counseled to avoid behavior, such as alcohol consumption, that may exacerbate liver damage. Two drugs in combination are recommended in a 24 to 48 week regimen. These are ribavirin (see chemotherapy section) and pegylated interferon alpha-2a and 2b (Peginterferon which has the trade names Pegintron (Schering-Plough) and Pegasys (Roche)). The latter consist of recombinant human interferon attached to polyethylene glycol which increases the half life of the protein in the bloodstream allowing weekly injection (rather than daily) and maintains a relatively constant level of the drug. Ribavirin has little effect on HCV alone but seems to enhance the effect of interferon. This two drug therapy results in the disappearance of HCV RNA from the blood of as many as 70% of patients and there is a marked drop in serum alanine aminotransferase levels; however, it is necessary to maintain the treatment as many patients relapse when the drugs are stopped. Those who exhibit a complete loss of HCV RNA both during treatment and afterwards are most likely not to relapse. The response depends on the genotype of the infecting virus. Unfortunately, this drug regimen many side effects.
After exposure immune globulin and interferon/Ribavirin are not recommended. In the case of needle sticks etc., the source should be tested for HCV and the patient referred to a specialist for management.
New Drug Treatments
New anti-hepatitis C drugs are currently being developed with the hope that the patient may be cured of the viral infection with 100% efficacy. As with current strategies against retroviruses, the aim of new anti-hepatitis C drugs is to target a specific viral enzyme.
In 2011, two new drugs were introduced, telaprevir and boceprevir. These inhibit the virus’s protease; however, they still required interferon and ribavirin. Their cure rate (no virus detectable after 12 weeks) is about 70 percent.
Sofosbuvir (Gilead Sciences), which will be available in 2013, inhibits the virus’s RNA polymerase enzyme. It is a chain-terminating nucleotide analog which is incorporated into newly synthesized viral RNA. Its
effectiveness varies according to which genotype of hepatitis C, infects the patient. About a quarter of patients in the United States are infected with hepatitis C genotypes 2 and 3. These patients are treated with sofosbuvir in combination with ribavirin but without interferon. Since interferon has to be injected, this will be the first completely oral treatment for hepatitis C.
More than 70% of patients infected with hepatitis C in the United States are infected with genotype 1. They require interferon plus ribavirin together with sofosbuvir over a period of 12 weeks. In a clinical trial, about 90 percent of previously untreated patients taking sofosbuvir in combination with interferon and ribavirin showed no detectable virus in the blood at the end of treatment.
HEPATITIS G
Like HCV, HGV is a flavivirus. Little is known about HGV but it can cause hepatitis, although it is probably not involved in a significant number of cases. In one survey in Japan, three of six patients with fulminant non-A, non-B, non-C, non-D, non-E hepatitis were positive for the presence of HGV genomic RNA sequences in their serum. HGV can produce mild acute infection and most infected people then go on to a persistent infection, which is probably not clinically significant. Transmission is via blood contamination such as transfusion or intravenous drug use. Hemodialysis is also a risk factor. Diagnosis is by antibodies or the detection of viral RNA by PCR.
GB virus (GBV, GB agent) was isolated in the 1960's from a surgeon (identified as GB) with acute hepatitis (from which he recovered) and was shown to cause hepatitis in tamarind monkeys. Two viruses called GBV-A and GBV-B were isolated from tamarind serum. A distinct, though similar, virus called GBV-C was found in a patient in West Africa. GB viruses are flaviviruses, like HGV. They have a 9400 nucleotide genome and sequencing shows that GBV-C has an 86% nucleotide similarity of HGV and a 96% similarity at the amino acid level. HGV and GBV are thus thought to be different isolates of the same hepatitis virus. Interestingly, co-infection of an HIV-positive patient with GBV seems to protect the patient from HIV. This happens because GBV infection results in a lower number of CCR5 molecules on the surface of the patient's T4 lymphocytes. CCR5 is a co-receptor (along with CD4 antigen) for HIV in human T-cells. Unfortunately, it appears that while a co-infection by HIV and GBV-C protects the patient, when the patient clears the GBV-C he/she is more likely to die of the HIV infection than someone with a persistent GBV-C infection. Moreover, infection by GBV reduces but does not eliminate CCR5 expression and there are other co-receptors that HIV can use to gain entry into a cell.
![]() Return to the Virology section of Microbiology and Immunology On-line
Return to the Virology section of Microbiology and Immunology On-line
This page last changed on
Friday, February 05, 2016
Page maintained by
Richard Hunt