Cryptococcus neoformans
and C. gattii (the Cryptococcus species complex)
Disease Definition
Exposure to cryptococci is common but disease
is uncommon and linked to host factors. The portal of entry is the
respiratory system. The patient acquires the infection from the
environment after inhaling airborne desiccated yeast cells and/or
basidiospores which then lodge in the lungs. Cryptococcosis manifests
most commonly as meningitis but many cases of pulmonary disease have
also been recognized. Another frequent site of dissemination is the
skin. Most infections are, however, asymptomatic. The disease is not
contagious.
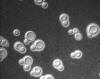
Etiologic Agents. Members of the Cryptococcus species complex include
Cryptococcus neoformans and C. gattii which are very distinctive yeasts.
The yeast cells are spherical, 3 - 7 µm diam., with narrow-based buds
and surrounded by a polysaccharide capsule (fig 13). They are classed in
the Basidiomycota and occur as two species complexes.
Background
C. neoformans was formerly considered a
single asexual species with 4 capsular serotypes (A,B,C,D.) Then, in
1970, a strain with distinct elliptical shaped yeast cells from an
African patient was named Cryptococcus neoformans var. gattii.
The observation of a sexual cycle by K.J. Kwon-Chung (1975) led her to
describe the sexual forms as Filobasidiella neoformans and
Filobasidiella bacillispora. Next, in 2002 C. neoformans var.
gattii was raised to species level. The sexual species F.
neoformans corresponds to asexual C. neoformans species
(serotypes A and D), and F. bacillispora corresponds to C.
gattii (serotypes B and C). Because the rule “One Fungus-One Name”
was adopted, the genus name “Filobasidella” no longer applies. Turning
to C. neoformans, phylogenetic analysis asserts that serotype A
is C. neoformans whereas serotype D is referred to as C.
deneoformans as the two species differ at ~10% of nucleotide positions.
Current consensus is there are two species complexes: Cryptococcus
neoformans species complex and the C. gattii species complex.
The status of C. gattii is unexpectedly genetically diverse.
Outbreaks of cryptococcosis in new geographic locations over the last 2
decades stimulated further molecular studies of C. gattii.
Phylogenetic Studies and
Molecular Subtyping
Molecular
typing analysis of C. gattii revealed intra-species genetic
diversity consisting of four distinct genetic groups: VGI-VGIV. This
nomenclature is used to apply to the global population structure of C.
gattii and enables tracing of virulent strains. From a practical
standpoint, it is not possible to identify these genetic species in the
laboratory since, at present, there are no certain biologic differences
and no differences in the rDNA genes commonly used for rapid DNA
testing. Consequently the recommended guideline, for the time being, is
to use “Cryptococcus neoformans species complex” and “C.
gattii species complex.” (Kwon-Chung et al., 2017)
In summary, the current concept of population structure of the
Cryptococcus neoformans and C. gattii species complexes is:
C. neoformans (molecular types VNI and VNII); C. deneoformans
(VNIV), C. gattii (VGI), C. bacillisporus (VGIII), C.
deuterogattii (VGII), C. tetragattii (VGIV), C. decagattii
(rare).
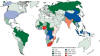
Geographic
Distribution/Ecologic Niche
The
geographic distribution of cryptococcosis is world-wide (fig 14.)
Cryptococcus neoformans
The ecological niche of C. neoformans
is pigeon and chicken droppings. Although this yeast is easily
recovered from pigeon droppings, a direct epidemiologic link has yet
to be established between exposure to pigeon droppings and a
specific human infection. Disease production is probably a property
of the host -- not the organism. The source of human infection is
not clear. This organism is ubiquitous, especially in areas such as
abandoned buildings contaminated with pigeon droppings.
Cryptococcus
gattii
This is found in soil and is associated
with several species of trees in tropical and sub-tropical regions
of the world. Recently, possibly as a result of climate change, C.
gattii infections were identified in the Pacific Northwest of the
U.S.A. and western Canada especially on Vancouver Island (see
Epidemiology Highlight, below and fig 15.)
Incidence/Prevalence
An estimated 220,000 cases of cryptococcal
meningitis occur among people with HIV/AIDS worldwide each year,
resulting in nearly 181,000 deaths. Most cases occur in sub-Saharan
Africa. In the U.S.A. estimates of cryptococcosis are incomplete because
the disease is only reportable in a few states. Results from
surveillance in two U.S. locations in the year 2000 indicated an annual
incidence of cryptococcosis among persons with AIDS was between 2 -7
cases per 1,000, with an overall incidence of 0.4 to 1.3 cases/100,000
cases (CDC 2017.)
C. gattii infections are worldwide, with cases of occurring in
Papua New Guinea, Australia, and South America. C. gattii infections
have also occurred in British Columbia, Canada since 1999 and in the
U.S. Pacific Northwest since 2004 (96 cases reported to CDC during
December 2004 – July 2011). Nearly all C. gattii cases in the
U.S. are from Oregon, Washington, and California with a small number in
other states.https://www.cdc.gov/fungal/diseases/cryptococcosis-gattii/statistics.html
- three
Epidemiology highlight
Emergence of
Cryptococcus gattii in the Pacific Northwest (Acheson et al.,
2017)
The ecology of C. gattii
remained unknown until its discovered association with Eucalyptus
trees in Australia in 1990. Cryptococcosis caused by C. gattii
was considered mainly a tropical and subtropical disease until
it emerged in 1999 on Vancouver Island, British Columbia, spreading
into the Pacific Northwest. Genomic studies traced the outbreak
genotypes, VGIIa and VGIIb, to an ancestral C. gattii strain
from the Amazon rainforest, spreading from there to different parts
of the world. Vancouver Island has one of the highest annual
incidences of C. gattii human infections in the world. From
1999 to 2015, 393 cases were reported in British Columbia (Acheson
et al., 2017.) The fungus infected immunocompetent individuals,
including local residents, visiting tourists, wild and domestic
animals. The search for its ecologic niche began in 2001. Cases were
clustered along the east side of Vancouver Island in the rain
shadow, where the flora and soil are unique to the Coastal Douglas
Fir and Western Hemlock biogeoclimatic zone of dry summers (average
17.6 ◦C), with mild winters rarely below freezing. Its emergence in
this temperate area suggests the fungus expanded its ecological
niche. Whether C. gattii was recently introduced to Canada or
existed undetected for years is not clear but a match between a
clinically isolated 1970’s Seattle strain and the 1999 Vancouver
Island outbreak VGII strain may suggest the latter.
Risk Groups/Risk Factors
As with other fungal infections, people at
most risk of cryptococcosis include people living with AIDS, and
patients receiving immunosuppressive therapy for cancer or for retention
of haemopoietic stem cell or solid organ transplants.
Transmission
The primary source of Cryptococcus
neoformans is soil mixed with excreta of the common pigeon, Columba
livia but pigeons, themselves, are not infected. Cryptococcus gattii
is found in the decaying hollows of certain tree species. Once
inhaled, desiccated yeast cells and/or basidiospores of cryptococci
germinate forming dividing yeast cells. They can then disseminate from
the lungs to other parts of the body via the bloodstream, sometimes
inside macrophages. The appearance of symptoms usually occurs several
months (average six to seven) after breathing in spores but, in some
cases, several years of latency pass before symptoms are observed.
Determinants of
Pathogenicity
The polysaccharide
capsule is antiphagocytic, may suppress T-cell function, and is
considered a virulence factor. C. neoformans also produces an
enzyme, phenoloxidase, involved in melanin production, another virulence
factor. The two species complexes’ ability to grow at 37oC differs from
the other saprobic Cryptococcus species, even those with polysaccharide
capsules.
Clinical Forms
Subclinical disease
and latency
The initial
exposure may be many years before the manifestation of disease, with
the yeast being sequestered during this time. When disease occurs it
may be subacute or chronic. In addition to causing meningitis, C.
neoformans may also infect lungs (figs 16, 17, 18) and disseminate
to the skin. The disease in the lungs and skin is characterized by
the formation of a granulomatous reaction with giant cells.
As with other fungal diseases, there
has been an increase in the recognition of pulmonary infection.
Pulmonary cryptococcosis is accompanied by malaise (fever and
headache), cough, shortness of breath, and chest pain. The yeast may
also form a mass in the mediastinum called a cryptococcoma. The
presence of Cryptococcus gattii can lead to the growth of
cryptococcomas in various parts of the body.
Cryptococcal meningitis
he fungus can spread from the lungs to the
nervous system, including the brain causing meningoencephalitis.
According to the CDC, there is a long latent time (two to fourteen
months) between exposure and the manifestation of symptoms. The
potentially fatal meningoencephalitis caused by C. neoformans has a
prolonged evolution over several months. Patients’ symptoms may
begin with vision problems and headache and, in the absence of
specific antifungal therapy, progress to delirium, nuchal rigidity,
then coma and death.
Symptoms include:
- Fever
- Headache
- Neck pain
- Nausea
- Light sensitivity
- Confusion
Therapy
The patient requires treatment by
antifungal agents for up to six months. The drugs of choice to
treat severe cryptococcosis are i.v .amphotericin B in
combination with 5-fluorocytosine (5-FC). 5-FC is an oral drug.
These two drugs are synergistic and their association is
advantageous. Stepdown therapy is the use of oral fluconazole.
In milder cases, or in resource-poor areas, fluconazole or
itraconazole is used as monotherapy.
Laboratory
Detection, Recovery, Identification
The alert physician will consider
cryptococcosis in the differential diagnosis and order a lumbar
puncture. The CSF is analyzed for its characteristic chemistry
(elevated protein and decreased glucose), cells (usually
monocytes), and evidence of an encapsulated yeast. The latter is
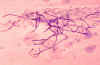
observed microscopically in an India ink prep, (fig 19) or by a
serologic assay for the capsular polysaccharide of C. neoformans.
The India Ink test demonstrates the capsule of this yeast,
whereas the lateral line assay for antigen (see below) is more
sensitive and specific such that a decreasing antigen titer
indicates a good prognosis, while an increasing titer has a poor
prognosis. When you consider cryptococcosis, think of capsules
and CNS disease.
Clinical material sent to the lab
is CSF, biopsy material, and urine (for some unexplained reason
the organism can be isolated from the urine in both the CNS and
systemic infections). This yeast will grow overnight on
bacterial or fungal media at 37°C. but growth is a little slower
at room temperature. In culture, the organism grows as creamy,
white, mucoid (because of the capsule) colonies. Growth in
culture is usually visible in 24 to 48 h. As the culture ages,
it turns brown due to melanin produced by the enzyme
phenoloxidase. After growth in the laboratory, microscopic
morphology is part of the laboratory identification of
Cryptococcus species.
Microscopy
The yeast is a round encapsulated
single cell with a narrow based bud. Overall size including the
capsule may be 15 - 20 µm diameter with a cell diameter of 7 µm.
Yeast cells vary considerably in size and shape. Identification
is based on physiologic reactions. The API20c profile is useful,
and the urease test is positive. Although they are white yeasts,
C. neoformans and C. gattii produce a brown colony
effect when grown on Niger seed agar or by using caffeic acid
disks. These tests are discussed in detail in Reiss et al.,
2012.
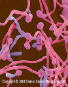
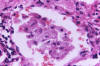
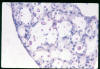
Histopathology
Pathologists use a mucicarmine
stain, which stains the capsule, to identify the organism in
tissue sections (fig 16). There is usually little or no
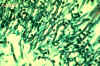
inflammatory response. The Direct Fluorescent Antibody test
identifies the organism in culture or tissue section causing the
yeast cell wall to stain green.
Serologic assays. Tests
for antibodies
To test the patient's serum there
are three serologic tests: The Indirect Fluorescent Antibody
test, the Tube Agglutination test for antibody, and antigen
detection in CSF or serum.
Tests for Antigen
The latex agglutination test, a
legacy test originated in the 1960’s (Bloomfield et al., 1963),
is being replaced by the IMMY CrAg LFA (Cryptococcal Antigen
Lateral Flow Assay). It is a rapid, immunochromatographic
dipstick test for the qualitative and semiquanitative detection
of cryptococcal polysaccharide antigen (IMMY, Inc., Norman OK.)
The CrAg LFA is sensitive and FDA cleared for both Cryptococcus
neoformans and C. gattii. It does not require
pretreatment of CSF by boiling or proteinase pretreatment of
serum. In the semiquantitative form two-fold serial dilutions
are set-up in individual tubes. A video of the method of
procedure is at :
http://www.immy.com/products/lateral-flow-assays/crag-lfa/#1473450453921-a2843b7f-7b86
As the patient improves, the
antigen titer will also decrease.
Preemptive antifungal treatment can
prevent or detect a significant proportion of cryptococcal
meningitis cases at an early stage because cryptococcal antigen
can be detected in blood before development of clinical disease.
The CrAg lateral flowassays that can be used for diagnosis at
the point of care. CrAg screening has been adopted into policy
by over 20 countries in Africa,
CGB medium
To distinguish Cryptococcus
gattii from Cryptococcus neoformans, the organisms
are grown on the differential medium:
canavanine-glycine-bromothymol blue agar (CGB.) C. neoformans
and C. gattii differ in their ability to assimilate
nitrogen. L- Canavanine is structurally related to L-arginine,
but when incorporated into proteins in place of arginine, there
is loss of function. C. gattii is resistant to canavanine.
C. neoformans is susceptible to canavanine or, if not, this
species does not assimilate glycine and the medium remains
yellow. On CGB medium C. gattii produces glycine decarboxylase
and uses glycine as a carbon and nitrogen source. The ammonia
released when glycine is cleaved causes an increase in pH
turning the indicator bromothymol blue from yellow to blue.
Given C. gattii's increasing profile worldwide as a
pathogen and its documented spread to nontraditional areas of
endemicity, CGB agar is an inexpensive and convenient way to
screen for emergence in patients.

 Figure 1
Figure 1 Figure 2
Figure 2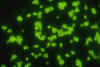 Figure
4
Figure
4 Figure 8
Figure 8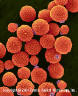 Figure 12
Figure 12