|
x |
x |
|
 |
 |
|
INFECTIOUS
DISEASE |
BACTERIOLOGY |
IMMUNOLOGY |
MYCOLOGY |
PARASITOLOGY |
VIROLOGY |
|
PORTUGUESE |
MYCOLOGY - CHAPTER ONE
INTRODUCTION TO MYCOLOGY
Dr Art DiSalvo
Emeritus Director, Nevada State Laboratory
Emeritus Director of Laboratories, South Carolina Department of Health and
Environmental Control
Dr Errol Reiss
Ph.D.
Research Microbiologist (retired)
Centers for Disease Control and Prevention
Atlanta, Georgia, USA
Dr Errol Reiss'
contribution to this Section is written in his private capacity. No
official support or endorsement by the Centers for Disease Control
and Prevention, Department of Health and Human Services is intended
nor should be inferred.
|
|
TURKISH |
|
ALBANIAN |
Let us know what you think
FEEDBACK |
|
SEARCH |
|
|
|
|
 |
|
|
 Figure 1
Figure 1
Mold (microscopic) Septate fungal hypha. Arrows point to septa
Credit: H.J. Shadomy
 Figure 2
Figure 2
Figure 2: Radiating hyphae of a mycelium
Credit: Dr. A.H.R. Buller, 1931
 Figure 3A
Figure 3A
Candida albicans growing as a unicellular budding yeast. Growth
at 37°C with aeration in yeast-peptone-dextrose broth medium. In this
image, unstained cells are magnified x400 (phase- contrast microscopy). |
CLASSIFICATION
Fungi defined
Fungi are simple eukaryotes with chitin-containing rigid cell walls and are
organized in the Kingdom Fungi. They do not contain chlorophyll and are not
plants. Medical mycology is mostly concerned with microfungi, specifically
zoopathogenic fungi. They grow in two forms:
Mold
A non-motile thallus constructed of apically elongating walled filaments (hyphae).
A web of filaments constitutes a mycelium (Figures 1 and 2)
Yeast (blastoconidia).
A unicellular fungus that reproduces by budding. Small, round projections
from the ellipsoid shaped parent cell are produced during mitosis followed
by migration of the nucleus and cytoplasm into the bud. Finally, cytokinesis
occurs forming a new daughter cell. Buds may be solitary or in chains.
Some yeasts multiply by fission. Events in the yeast cell cycle are
finely orchestrated. A visual 3-D representation of the yeast cell cycle can
be found
here. Candida albicans is a yeast-like fungus that grows in a variety of
forms: yeast, pseudohyphae (a transitional form) and hyphae. Pseudohyphae
can give rise to yeast cells by apical or lateral budding. Yeast can also
convert to a hyphal form. All three forms are found in tissue invaded by the
fungus. Figures 3 A - C show C. albicans growing as a unicellular budding
yeast under some environmental conditions and as a filamentous fungus under
other conditions.
Figure 4 shows a colony of yeast and of a mold growing in agar plate
cultures.
WHERE DO FUNGI GROW?
Most are saprobes that decompose dead organic matter. In contrast to plants
and algae, fungi are “heterotrophs”: they cannot make their own food and instead
obtain it by uptake of organic matter. Plant pathogenic fungi cause damage to
food crops, trees, and other plants. Some fungi are “commensals” living on the
mucous membranes and skin of mammalian hosts. Various estimates of the number of
fungal species range upwards of 1 million (Heitman, 2011). About 300 species are
known human pathogens but any fungus capable of growth at 37 degrees C is potentially
pathogenic in a suitably compromised host.
|
|
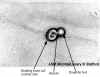 Figure 3B
Figure 3B
At higher magnification a budding yeast is seen with a
septum formed between the daughter bud and the mother cell. The
unstained cell is magnified x1,000 (phase-contrast)
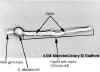 Figure 3C
Figure 3C
At 37degrees C a C. albicans yeast cell is shown
germinating. (i.e., forming a germ tube). This then grows into a
filament (hypha) with a septum between cells. (x1000).
Figure 3 A-C
© Phillip Stafford
Dartmouth Medical School
Hanover, New Hampshire and
The
MicrobeLibrary |
FUNGI DIFFER FROM BACTERIA IN THEIR
ORGANELLES AND METABOLISM
Capsule
Polysaccharide capsules of bacterial pathogens are virulence
factors. A major fungal pathogen, Cryptococcus neoformans (also
C. gattii). has such a capsule.
Cell walls
Bacterial cell walls contain peptidoglycan, lipopolysaccharide, and
teichoic acid. Fungal walls contain glucan, mannan, and chitin.
Cytoplasmic membranes
Membranes of fungi contains ergosterol, not present in bacteria. The
synthesis of and binding to ergosterol are potent antifungal drug
targets.
Episomes and plasmids
Bacterial resistance to antibiotics is mediated by extrachromosomal
DNA; no such mechanisms are known to exist in fungi.
Nucleus
As eukaryotes, fungal genes are organized into chromosomes, enclosed
in a nuclear membrane. Baker’s yeast, Saccharomyces, has 16
chromosomes. Bacteria, as prokaryotes, have a single chromosome, not
enclosed by a membrane, but packed into part of the cytoplasm, the
nucleoid, occupying ~1/3 of the cell volume.
Ribosomes
Bacteria 30s + 50s form 70s ribosomes; fungi 40s + 60s form 80s
ribosomes
Dimorphism
Some fungi undergo morphogenesis into two forms, such as yeast and
mold forms. This feature is absent in bacteria
- Metabolism
Bacteria are aerobic or anaerobic; fungi during tissue invasion of
humans are aerobic, but metabolism by fungi under anaerobic conditions
is known, e.g., fermentation by Saccharomyces beer yeast occurs at low
oxygen concentrations. Energy transduction in bacteria occurs at the
cell membrane; in fungi mitochondria perform this function.
- Reproduction
Bacteria reproduce by binary fission to two identical daughter cells.
Fungi reproduce in various ways: budding, linear extension of the
growing tips of hyphae, and by the production of various types of
spores, which in fungi are called conidia.
- Size
The volume of a typical bacterium, E. coli , is 1 µm3, diameter 1 µm,
and length ~2 µm.
See
here.
In contrast, A budding yeast cell has a V = 42 µm3 (haploid
strain) and V= ~82 µm3 (diploid strain), diameter 3-6 µm. See
here.
These differences in cellular organization help explain why
antibiotics active against bacteria are, with exceptions, inactive
against fungi. On the other hand, similarities between the
organization and metabolism of fungal cells and human cells
complicates development of antifungal agents with selective toxicity
for fungi.
|
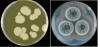 Figure 4
Figure 4
Yeast (colony) and Mold (colony)
Left: Agar plate with yeast colony. Candida albicans growing on
SABHI agar. Credit: Dr. William Kaplan, CDC.
Right. Agar plate with mold colony: Aspergillus fumigatus.
Credit: Mr. Jim Gathany, CDC Creative Arts Branch.
|
|
|
| |
CLASSIFICATION
There are two types: Biological classification and classification based on
the primary site of pathology. Students of medicine will find the second type of
classification most useful.
Classification of Fungi based on the
Primary Site of Pathology
Superficial mycoses
This category is typified by pityriasis versicolor, caused by Malassezia
species. This yeast grows on the non-living keratinized outer layer of the
skin of humans and dogs also includes dandruff and other forms of seborrheic
dermatitis, rarely the cause of invasive disease.
Cutaneous mycoses
Dermatophytosis, also known as ringworm, is caused by Trichophyton
and Microsporum species. They are restricted to grow on the
non-living keratinized outer layer of skin of humans, dogs, and cats.
Medical terminology assigns a name to the diseases according to the body
site affected: Tinea capitis is scalp ringworm, tinea cruris is jock itch,
tinea unguium fungal nail infections, etc. A completely different category
is the cutaneous site of disseminated mycoses. Skin is a frequent site for
disseminated blastomycosis.
Opportunistic mycoses
Opportunistic yeasts and Pneumocystis
Candidiasis includes mucocutaneous and deep seated disease caused by C.
albicans and non-albicans Candida species yeasts. Their ecologic niche
is the skin and mucosae of warm-blooded animals and humans. Cryptococcal
meningo-encephalitis is caused by the environmental yeasts Cryptococcus
neoformans and C. gattii. Pneumocystis pneumonia (“PCP”) is caused by
Pneumocystis jirovecii, an obligate endogenous commensal of the human
lung.
Opportunistic mold disease
Disease is encountered in debilitated or immunocompromised hosts. The
causative agents are non-pigmented molds that are ubiquitous in the
environment and cause disease when their conidia are inhaled by a
susceptible host or when the conidia alight on skin of burn patients or
on wounds. Invasive pulmonary aspergillosis is caused by Aspergillus
fumigatus and related species. Mucormycosis is caused by various
Mucorales species especially Rhizopus oryzae (syn: R. arrhizus)
including rhinocerebral mucormycosis occurring in diabetic ketoacidosis
(figure 5). Fusarium species mycosis includes sino-pulmonary-disseminated
disease and, in immune normal persons, keratitis (due to penetrating
injury or contaminated contact lenses.) Scedosporium species cause
pulmonary disseminated disease and, in immune-normal persons, eumycetoma
most often resulting from injury during barefoot labor.
Subcutaneous mycoses of implantation
Subcutaneous mycoses are confined to the subcutaneous tissue and
systemic spread is rare. Following a penetrating injury with thorns,
splinters these agents can develop into deep, ulcerated skin lesions,
subcutaneous cysts, or slowly enlarging warty masses.
Sporotrichosis is the most common human subcutaneous mycosis,
worldwide in its distribution, also affecting cats. It is caused by
the dimorphic fungus Sporothrix schenckii.
Melanized fungi (formerly referred to as “dematiaceous”) cause a
spectrum of disease separated into three categories:
Chromoblastomycosis described as warty, slow growing tumor-like
cutaneous-subcutaneous masses caused by Fonsecaea pedrosoi, among
other species, with the characteristic dimorphic tissue form of
round copper-colored muriform cells.
Phaeohyphomycosis is a term derived from the histopathologic
appearance of the fungi in cutaneous-subcutaneous cysts: dark
yeast-like, pseudohyphae-like, or variously shaped hyphae or a
combination of forms. (”Phaeo” from the Greek=dark.) Exophiala
dermatitidis formerly “Wangiella dermatitidis” accounts for
approximately 30% of human isolates in the U.S.A. (Zeng et al.,
2007.)
Eumycetoma. The hallmark of this mycosis is a triad of tumefaction,
swelling, and sinus tracts draining “grains” (masses of fungal
hyphae) occurring mostly on the extremities, i.e., “Madura foot”.
Causative agents are many including Scedosporium and Madurella
species. Exposure follows puncture wounds during barefoot labor in
the endemic tropical and subtropical areas of the world.
|
|
|
DIMORPHIC FUNGI
Some fungi have two growth forms such as certain soil-dwelling molds that are
primary respiratory pathogens. Their conidia become airborne and, when inhaled,
can survive and undergo morphogenesis to the pathogenic yeast form at 37 degrees C.
Specimens, such as sputum, when plated on mycologic medium and incubated at
30 degrees C, grow as molds. This category includes the commensal yeast, Candida
albicans which in tissue invasion may assume conformations of yeast,
pseudohyphae and true hyphae. Dimorphism in fungal pathogens includes
Coccidioides species, filamentous in the environment, converting to
endosporulating spherules in the human or animal host.
Endemic Mycoses caused by Dimorphic
Environmental Molds
Several soil-inhabiting saprobic fungi have gained the capacity to
parasitize mammals causing systemic infection in immune-normal individuals.
Disease occurs in defined geographic areas, following inhalation of conidia,
beginning asymptomatically in the lungs and progressing to an influenza-like
illness or pneumonia. Once inhaled these agents convert from mycelial to
yeast or spherule form in the host. If the inhaled dose of conidia is high
and the host immune response is insufficient extrapulmonary dissemination
can ensue.
Persistence, dormancy, and reactivation may also occur. The agents are
Blastomyces dermatitidis, Histoplasma capsulatum, Paracoccidioides,
brasiliensis, P. lutzii, Lacazia loboi, Coccidioides
immitis, C. posadasii, Talaromyces (formerly
Penicillium) marneffei. Each genus has its own predilection for
various organs which will be described in discussing the individual
diseases.
Coccidioidomycosis
Coccidioidomycosis has two major endemic areas in the U.S.; the
California endemic area centered in the San Joaquin valley and the
Arizona endemic area centered in Maricopa County including Phoenix and
Pima County including Tucson.
Histoplasmosis
The endemic area for histoplasmosis is along the river valleys of the
central U.S., overlapping with the blastomycosis area which extends into
Canadian provinces bordering the Great Lakes and northern Ontario.
Paracoccidioidomycosis
This is a rural disease endemic to Mexico, and Central and South
America, especially in coffee growers of Colombia, Venezuela, and
Brazil. Lobomycosis is encountered in the Amazon Rain Forest ecosystem
and is transmitted by traumatic lesion from splinters or bites of
insects, snakes, rays.
Sporotrichosis
This is also considered in this group, since it is dimorphic, although
its geographic distribution is world-wide, there are highly endemic
areas in Brazil, India, Mexico, Japan, Peru, Uruguay and South Africa.
Sporothrix schenckii ecologic niche and route of infection are different
in that transmission occurs through traumatic implantation from thorny
plants, wood splinters, sphagnum moss, and hay.
Talaromycosis
Talaromyces (was Penicillium) marneffei is endemic
in SE Asia, especially in Thailand, also in Southern China and Hong
Kong. Alone among these endemic mycoses T. marneffei rarely
infects immune normal humans.
|
|
|
|
|
|
|
| |
MORPHOLOGY In addition to the
yeast and mold growth forms referred to above, intermediate forms exist as
“pseudohyphae” in Candida albicans.
MYCOTIC DISEASES
There are four types of mycoses:
- Hypersensitivity. An allergic
reaction to molds and their airborne conidia.
- Mycotoxicoses. Poisoning of humans and lower animals by ingestion of
food or feed contaminated by low molecular wt fungal toxins produced by
pre-harvest infestation or during storage of peanuts, grains (Pitt and
Miller 2016).
- Mycetismus. Poisoning after ingestion of certain mushrooms (50-100
cases/year in U.S.A). (Smith and Davis 2016.)
- Infection. Inhalation, ingestion, or implantation of infectious
propagules that progresses to disease via tissue invasion, evoking a host
immune response. We shall be concerned only with the last type: disease
resulting from infection with pathogenic fungi.
Host and Microbial Factors affecting
Pathogenicity
Host Risk Factors (Muskett et
al., 2011)
Patients receiving immunosuppressive therapy for maintenance of a
transplanted organ or stem cell transplant, cancer chemotherapy,
autoimmune disease, and in persons living with HIV/AIDS.
- Prolonged ICU stay, mechanical ventilation.
- Very young (< 1 mo.) or aged (>65 y) patients
- Inborn or acquired deficits: chronic granulomatous disease,
cystic fibrosis, diabetes.
- Invasive diagnostic and surgical procedures: abdominal surgery,
prosthetic implants, indwelling catheters, renal dialysis.
- Travel to or residence in an endemic area.
- Occupational or recreational exposure: Barefoot labor, gardeners
exposed to thorny plants, workers in demolition of old buildings.
Microbial Factors
Elucidation of mechanisms of pathogenicity is an active area of
research. The following are a sampling of our understanding of factors
affecting fungal pathogenesis.
- Adhesins. Adherence to endothelial cells is a prime requisite
for tissue invasion. Examples of fungal adhesins are: ALS of C.
albicans, BAD1 of Blastomyces dermatitidis.
- Biofilm formation on biomaterials. Ability of fungi to adhere to
and embed in biofilms increases their resistance to antifungal
agents.
- Capsule. Encapsulated microbes are resistant to phagocytosis and
are implicated in CNS disease. Among fungi, Cryptococcus
neoformans has an acidic high molecular wt polysaccharide
capsule that is antiphagocytic and may facilitate endothelial
crossing into the CNS (Zaragoza et al, 2009).
- Melanin. Melanin in fungal cell walls makes them resistant to
phagocytosis and killing. Some examples of melanized fungi include
Cryptococcus neoformans, Paracoccidioides brasiliensis,
Sporothrix schenckii (Nosanchuk et al., 2006).
- Resistance to the oxidative burst of polymorphonuclear
neutrophilic granulocytes. Primary respiratory pathogens, e.g.:
Blastomyces, Histoplasma, Paracoccidioides, and
Sporothrix, but not opportunistic fungi, e.g.: Candida
species, can resist the effects of the active oxygen radicals
released during the respiratory burst (Schaffner A et al., 1986).
- “Shape shifters”. Ability to grow in different tissue forms
facilitates tissue invasion. e.g.: Histoplasma is dimorphic
and the yeast forms multiply within host macrophages.
- Thermotolerance. Pathogenic fungi can grow at 37oC.
Using these stratagems, fungi are able to withstand host defenses.
Fungi are ubiquitous in nature so that human and animal exposure is
common but disease is uncommon and linked to: (a) host factors, outlined
above, and (b) in the case of primary respiratory pathogens the inhaled
dose of infectious propagules.
Example. The demonstration of fungi in blood drawn from an
intravenous catheter may correspond to colonization of the catheter,
to transient fungemia (i.e., dissemination of fungi through the
bloodstream), or to a true bloodstream infection. The physician must
decide which fits the clinical status of the patient based on
physical examination, laboratory tests, and imaging studies. The
decision to treat is not trivial, because systemic fungal infections
require the aggressive use of drugs, some with considerable
toxicity.
Are fungal diseases communicable? Most mycotic agents are soil
saprobes and mycoses are generally not communicable from
person-to-person. Some exceptions are dermatophytes: Tinea pedis
exposure in gym locker rooms, scalp ringworm in young school children;
oropharyngeal or vulvovaginal candidiasis and, probably, colonization
with Pneumocystis jirovecii.
Mycotic disease outbreaks
Outbreaks occur when the environment containing primary respiratory
pathogens is disturbed. These fungi have a particular, characteristic
ecologic niche in nature. In this environment, the normally saprobic fungi
proliferate and develop, providing a source of fungal elements and/or
conidia, to which humans and animals, the incidental hosts, can become
exposed. Illustrations are:
- Coccidioidomycosis among more than 300 participants of the World
Championships of Model Airplanes, held each year in Lost Hills, Kern
County, Calif., a highly endemic area.
(Centers for Disease Control and Prevention, CDC, 2001).
- Histoplasmosis. At least 36 persons attending a Lung Association
event in November 2007 at the Iowa Governor’s mansion contracted
histoplasmosis. Bat droppings were found in the mansion's attic, in at
least one other room, and in air filters. Bird and bat nesting sites and
their guano are a known source of Histoplasma conidia.
- Blastomycosis. Six cases of blastomycosis occurred among 2 groups of
4th-6th grade students visiting a beaver pond at an environmental camp
at Eagle River, Wisconsin in 1984. Cultures of soil from the beaver
lodge and rotten wood near the beaver dam yielded Blastomyces
dermatitidis (Klein et al., 1986).
- In recent years outbreaks of mycoses have accompanied the use of
contaminated injectable medicines, insufficiently antiseptic contact
lens cleaning and storage solutions.
The physician must be able to elicit a complete history from the patient
including occupation, avocation, and travel history. This information is
frequently required to raise or confirm a differential diagnosis.
|
 Figure 8
Figure 8
Histopathologic section shows Histoplasma capsulatum yeast forms
in tissue. The Gomori methenamine silver stain with green counterstain
does not show tissue reaction.
Credit. Dr. William Kaplan, CDC |
DIAGNOSIS Types of specimens
received for direct examination and culture are similar to those submitted for
bacteriology.
Specimen Processing
Chances to recover fungi are increased, with bacterial growth minimized,
when clinical specimens reach the laboratory within 2 h of collection.
This guide applies especially to urine specimens, which also may be
stored at ≤ 24 h at 4 degrees C. Exceptions are that hair, nails, and skin
scrapings may be stored up to 72 h at room temperature before culture,
and they may be shipped by mail. CSF specimens are stored at 30oC and
never refrigerated because CSF is a good culture medium and fungi will
continue to replicate at room temp or 30oC.
Microscopic detection is facilitated when wet mounts of certain
specimens are digested with 10% KOH ± Calcofluor: skin scrapings, hair,
nails, corneal scrapings, and wound exudates. Visibility of fungal
elements is increased when wet mounts are combined with fluorescent
brightener, Calcofluor. Sputum specimens may require digestion with
Mucolyse® (dithiothreitol in phosphate buffer) to reduce viscosity.
Tissue obtained by biopsy or surgery is prepared for culture by grinding
or homogenization if Histoplasma is suspected, but for other fungi and
Mucorales species, mincing and/or finely slicing are preferred so as to
not disrupt hyphal elements.
Histopathology
Formalin fixed, paraffin embedded (FFPE) tissues are treated with
special fungal stains, or subject to fluorescent antibodies, to reveal
fungal elements. The H&E stain does not always tint the organism, but it
will stain inflammatory cells. The Gomori methenamine silver (GMS) stain
is used to reveal fungi which stain black against a green background
(figure 8). A combined H&E-GMS stain variation will demonstrate both
fungi and the inflammatory response. See
here.
|
 Figure 9
Figure 9
Asci (Cysts) of Pneumocystis jirovecii in lung tissue, stained with
Gomori methenamine silver and hematoxylin and eosin (H&E). The walls of
the cysts are stained black. (source CDC DPDx) |
Figure 9 shows asci (Cysts) of Pneumocystis jirovecii in lung
tissue, stained with methenamine silver and hematoxylin and eosin (H&E).
The walls of the cysts are stained black.
Other special fungal stains for FFPE tissue
sections
- Periodic Acid Schiff is a good general stain for fungi, and can
be counter-stained with hematoxylin to demonstrate tissue reactions.
- Mucicarmine will stain the capsule of Cryptococcus species.
- Fontana Masson stains fungal cell wall melanin.
- Fluorescent antibodies may be used for microscopy on fixed
tissue sections but commercial reagents are scarce. One good example
is the Merifluor® Pneumocystis kit (Meridian Bioscience, Inc.)
- FISH. Fluorescent in situ hybridization has found an application
in smears of blood cultures. Fluorescent labeled peptide-nucleic
acid probes are hybridized to fungal RNA. The AdvanDX® Peptide
Nucleic Acid in Situ Hybridization Yeast Traffic Light system is
available for microscopic detection of Candida species directly from
blood culture bottles.
Culture
Definitive diagnosis requires culture and identification. Emmons
modification of Sabouraud dextrose agar (SDA-Emmons) is extensively used for
primary isolation of pathogenic fungi but Mucorales, black molds,
dermatophytes, and yeast have higher percentage recoveries when plated on
inhibitory mold agar (Scognamiglio et al., 2010). Cycloheximide may be added
to suppress saprobic fungi but then a medium without cycloheximide should be
paired. Chloramphenicol is often included to inhibit bacterial growth. If
Histoplasma or Blastomyces are suspected an enriched medium such
as brain-heart-infusion agar is indicated, bearing in mind that media
supplemented with cycloheximide will inhibit the yeast form of these
dimorphic fungi. There are many other formulations of mycologic media.
Sources of such formulations can be found
here. This site is originated by Prof. Lynne Sigler of the University of Alberta Microfungus Collection and Herbarium, Canada. The Difco™ & BBL™ Manual of
Microbiological Culture Media” (2nd Ed.) can be viewed and downloaded from
the bd.com website.
Cultures of primary systemic dimorphic fungi, (e.g.: Blastomyces,
Histoplasma) are identified in the laboratory by (a) slide culture
and/or tease mount methods revealing characteristic microscopic morphology
and (b) importantly by DNA probe tests of their mold forms grown at 25-30
degrees C
(AccuProbe, Hologic Inc., San Diego, CA), (c) Recently MALDI-TOF (see below)
has become an important, rapid, and reliable tool for identification.
Fungi grow best at 30 degrees C but if that temp is not available 25 degrees
C will suffice. It is unnecessary to incubate an additional culture at 37 o
C unless there is reasonable suspicion of a thermally dimorphic fungus.
Cultures are not considered negative for growth until after 4 weeks’
incubation. Once a pure culture is obtained identification is made by
observing colony and microscopic morphology for molds. Rapid tests for yeast
identification are summarized in Reiss et al., 2012 section 2.3.7.2.
|
| |
Identifications based on DNA
sequence and proteomics analysis
The field of medical mycology reliant on classical morphologic diagnostic
methods is now fully engaged in automated sample processing and walk-away
instrumental analysis leading to a diagnosis from either isolated colonies
or, identification directly from patient blood samples or from positive
blood cultures. Such methods are expensive, but often do not require highly
trained technologists, and shorten the time to a result to hours, or even
less time.
MALDI-TOF MS
Matrix Assisted Laser Desorption/Ionization-Time-of-Flight-Mass
Spectrometry. With minimal sample preparation from small isolated colonies
MALDI-TOF MS produces a spectrum of protein fragments of known molecular
mass. The resulting peptide mass fingerprint leads to faster, even same day,
clinical decisions. When an incubated sample becomes a visible colony (~ 105
cfu) it can be subject to laser analysis, taking less than 10 min. An
organism ID can then be made with little or no reduction in reliability. An
end-to-end system including a database is the BioTyper® (Bruker Daltonics).
Commercial Multiplex PCR Assays for
rapid detection of fungal infections
MicroSeq® Rapid Microbial Identification System (Life Technologies Inc.)
combines reagents, instrumentation and a database of curated D2 18S rDNA
sequences for upwards of 800 yeasts and molds. Alternative databases exist
e.g.: MycoBank at the International Mycological Association, and GenBank at
the National Center for Biotechnology Information (NCBI).
Luminex® xMAP Fungal Assay. This is a PCR micro-bead probe fluid array (Luminex
Molecular Diagnostics) that detects Candida species (C. albicans, C.
glabrata, C. tropicalis, C. parapsilosis, C. lusitaniae, C. guilliermondii,
and C. krusei) or a mold 11-plex panel. To understand the method please view
the
MAGpix video.
FilmArray® (BioFire Diagnostics, A bioMérieux Company) is an FDA approved
multiplex PCR with integrated sample preparation and automated analysis that
detects 5 Candida species in positive blood cultures: Candida albicans, C.
glabrata, C. krusei, C. parapsilosis and C. tropicalis. Sample from a
positive blood culture is injected into a sample pouch and then the process
then is automated and, including sample preparation, takes approximately 2
min to a result. Software calls and reports either a positive or negative
for each microbe in the array blood panel. Detection is via a fluorescent
dye binding to double stranded DNA. Microbes are identified depending on
which well in the film array is positive. An
illustrative video
explains the method.
T2 Candida (FDA approved) (T2 Biosystems) Magnetic resonance technology is
used to detect PCR amplicons of Candida DNA isolated from whole blood. Cells
from 5 different Candida species in whole blood can be detected without the
need to incubate blood samples. The T2Dx® instrument is automated:
extracting DNA from whole blood followed by PCR amplification of rDNA.
Detection is via nanoparticles coated with DNA probes complementary to
different Candida species. A signal is generated and detected by T2 magnetic
resonance. Nanoparticles with superparamagnetic properties increase magnetic
resonance signals. These particles are coated with Candida species-specific
probes. NMR spectrometers are available as benchtop models. The assay
identifies Candida albicans and/or C. tropicalis, C. parapsilosis, and C.
glabrata and/or C. krusei. The test does not distinguish between C. albicans
and C. tropicalis. The test does not distinguish between C. glabrata and C.
krusei. Details of the method may be
viewed
in the submission to the U.S.
FDA.
IRIDICA assay (IBIS/Abbot Molecular) (not available in the USA). This
multiplex PCR method directly detects microbial DNA in blood. It is capable
to detect C. albicans, C. glabrata, C. parapsilosis and C. tropicalis. This
system is not available in the U.S.A. The PCR target is large subunit rDNA
for ~200 fungal species.
Automated sample processing extracts DNA from 5 mL of whole blood. Hundreds
of diverse microbes are identified from the species-specific genetic
signatures in the PCR fragments using electrospray ionization mass
spectrometry (PCR/ESI-MS). A computer parses and reports detection of 673
species of bacteria and Candida on the basis of multi-locus amplicon base
composition signatures. The method can identify microbes from uncultured
blood in less than 8 h.
Blood cultures
Detection of fungemia is an essential part of laboratory identification. A
positive blood culture requires an immediate report to the physician of a
presumptive fungal infection, with specific identification to follow. Two
general types of blood culture methods are in use: manual and automated.
Manual methods consist of broth, broth with an agar layer, or a solid agar
medium. Examples of manual methods are (1) A biphasic broth/agar
combination, the Septi-Chek® paddle device, using slide agars immersed in
broth (BD Diagnostic Systems, Sparks, MD). (2) The Isolator® solid medium,
system (Alere N.A., Waltham MA) is a single tube test combining lysis and
centrifugation: lysis of RBC and WBC releases phagocytosed yeast forms of
dimorphic fungi, then centrifugation through a dense fluorocarbon cushion
produces a sediment which is planted to agar medium.
Automated continuously monitored blood culture systems. There are choices of
such systems which have become the standard method in large medical centers.
They are based either on the emission of CO2 during respiration of fungi or
measurement of head gases above the surface of the broth blood culture
bottle.
Blood culture bottles in the BacT/ALERT® (BioMérieux Corp.) system change
color when the pH changes with increasing CO2 concentrations. The BD Bactec™
FX blood culture (BC) system has dye in the sensor at the bottom of the vial
which reacts with CO2 released by microbial growth. This modulates light
absorbed by a fluorescent material in the sensor. A photo detector then
measures fluorescence and turns “on” indicating growth in the bottle.
VersaTREK™ is another automated system that measures head gas changes in the
blood culture bottles when there is growth.
Serology
Antibody tests
Serology may be helpful when applied to a specific fungal disease. The
efficacy of serology varies with different mycoses. The serologic tests
will be discussed under each mycosis. The most common serologic tests
for fungi are based on double immunodiffusion, complement fixation and
enzyme immunoassays (EIA). Double immunodiffusion and complement
fixation usually detect IgG antibodies. Some EIA tests detect both IgG
and IgM antibodies.
Antigen Detection
Several antigen detection methods have received good confirmation in
clinical practice and are generally available in the U.S.A.
- Cryptococcal antigen test in CSF, plasma, or serum (CrAG® LFA, IMMY
Corp, Norman, OK). Uses a lateral flow “dipstick” test and is rapid,
sensitive and, with sample dilutions, can indicate a titer.
- Galactomannan antigenemia is detected in invasive aspergillosis (Platelia™
Aspergillus EIA, Bio-Rad.com). See
here.
- Histoplasma polysaccharide antigen test (HPA) is a good
indicator of active invasive or disseminated histoplasmosis.
MiraVista
Diagnostics, Indianapolis IN
- Screening serum test for fungi in general: (1→3)-ß-D-glucan
detection (Fungitell® Assay, Associates of Cape Cod Inc.) Exceptions are
the Fungitell assay does not detect this polysaccharide in fungi in the
genus Cryptococcus, or in the order Mucorales.
Skin testing (dermal
hypersensitivity)
This was popular as a diagnostic tool, but is now discouraged because
the skin test may interfere with serologic studies, causing false
positive results. It may still be used in clinical immunology to
evaluate the patient's immune status and, where reagents are available
and approved for use, as a population exposure index in epidemiologic
studies.
|
|
MOLECULAR STRUCTURE
Amphotericin B
Ketoconazole
Griseofulvin
5-fluorocytosine
MOLECULAR
STRUCTURE
Ergosterol
Caspofungin |
TREATMENT
This introduction to antifungal agents describes the class of the agent, its
mode of action, and a summary of its action spectrum. Further information is
included in discussion of the individual mycoses. Structure diagrams for
antifungal agents can be found
here.
Although one of the first anti-infective agents (oral iodides) was an anti-mycotic
first used in 1903, the development of antifungal agents lagged the development
of antibiotics against bacteria. Discovery of compounds with selective toxicity
for the invading fungus avoiding serious adverse effects to the host has proved
difficult because mammals and fungi are both eukaryotic, with similar cellular
organelles and biochemical pathways. For example, an important drug target is
membrane sterols, ergosterol in the fungal cell membrane and cholesterol in the
mammalian cell membrane. Binding of drug to the ergosterol pathway carries risk
for concomitant damage to host membranes. Fortunately, the armamentarium now has
more choices and less toxicity.
Polyene antimycotic agents
The natural products, nystatin and amphotericin B (AmB), were co-discovered
in 1953 from a Streptomyces nodosus soil isolate from Venezuela. Structure
analysis revealed AmB has 7 conjugated double bonds linked to an amino
sugar, mycosamine. It is “amphipathic” with a lipophilic face and a
hydrophilic face, the latter containing 6 hydroxyl groups. The 2-faced
structure is important in its mode of action. AmB is potent and fungicidal
but is light labile and water-insoluble. Squibb laboratories in 1958 devised
a way to obtain a suspension with sodium deoxycholate solution. The
lyophilized product added to a glucose solution forms a micellar suspension
that can be infused into patients. Nystatin is too toxic for IV use and is
reserved for mucosal and skin applications. AmB has acute effects after IV
administration: thrombophlebitis, fever, chills, nausea. Because of these
and dose-limiting nephrotoxicity, in the 1980s AmB was incorporated into
liposomes consisting of dimyristoyl phosphatidylcholine and dimyristoyl
glycerol in a lipid-drug wt ratio of 12:1 to form liposomal AmB. This
formulation has reduced nephrotoxicity and fewer infusion-related reactions.
Other polyenes were discovered but aside from these two only pimaricin (natamycin)
is in clinical use.
Mode of action. AmB binds to ergosterol in the fungal membrane producing ion
channels through which cell contents leak resulting in cell death. A
secondary mode of action occurs from auto-oxidation of AmB inducing
oxidative stress that may contribute to rapid fungicidal activity.
Action spectrum. The major applications for AmB and liposomal AmB are
invasive candidiasis, cryptococcosis, mucormycosis, and treatment for
mycoses caused by dimorphic endemic fungal pathogens.
Azole antifungal agents
These are chemically synthesized based on the imidazole structure, moving
later to triazoles with broader action spectra. Clotrimazole and miconazole
were introduced in the late 1960s. Adverse effects and unpredictable
pharmacokinetics limit clotrimazole to topical treatment. Miconazole is a
useful topical agent but toxicity limits its parenteral use. Ketoconazole is
an imidazole which, for almost a decade, was the only available oral agent
for systemic mycoses. Shortcomings were inter-patient variation, poor CNS
penetration, fungistatic nature, with adverse effects including drug-induced
hepatitis, inhibition of testosterone and cortisol. Moreover, it was
associated with poor response rates and recurrences of major mycoses. The
era of triazole antifungal agents began with fluconazole in 1990. It is
water soluble, can be delivered IV and, after oral administration,
absorption is almost complete. It enters the CSF with concentrations nearing
80% of serum levels. Fluconazole came into wide use for mucosal and invasive
yeast infection. The need for a broader spectrum azole was met in 1992 with
the introduction of itraconazole, active against dimorphic endemic fungi and
Aspergillus. The debut of extended spectrum triazoles followed, voriconazole
in 2002 (based on the structure of fluconazole) and posaconazole in 2006
(similar in structure to itraconazole).
Mode of action. Azoles inhibit ergosterol biosynthesis at the C-14
demethylation stage. Through their azole ring, they form a complex with the
heme iron of P-450 demethylase. Depletion of ergosterol results in cell
membrane damage with collateral damage to membrane-bound enzymes active in
nutrient transport, chitin synthesis, and growth. Azoles are fungistatic
against yeast species, e.g.: Candida and Cryptococcus, but other triazoles
appear fungicidal against Aspergillus spp.
Action spectrum. Fluconazole is active against most Candida and Cryptococcus
spp., Histoplasma capsulatum, Coccidioides spp., and Paracoccidioides
brasiliensis. It is in general use for maintenance therapy of AIDS-cryptococcosis.
Itraconazole is used with good results against yeast and molds especially
dermatophytes, Sporothrix schenckii, and for endemic dimorphic pathogens,
against non-life threatening, non-meningeal forms of disease. Voriconazole
is recommended for primary treatment of invasive aspergillosis; with AmB and
liposomal AmB reserved for initial and salvage therapy where voriconazole
cannot be used. Voriconazole is important in treating Fusarium mycosis, as
well as AmB-resistant species: Aspergillus terreus and Pseudallescheria
boydii. Posaconazole is active against opportunistic molds including the
difficult to treat Mucorales and is approved for prophylaxis of invasive
fungal infections in stem cell transplant recipients, and those with
hematologic malignancy and prolonged neutropenia. Owing to the development
of these triazoles, the treatment of invasive fungal infections is no longer
limited by acute toxicity.
5-fluorocytosine (5-FC)
This is rarely used as monotherapy because of rapid development of secondary
resistance. Combined with AmB it is useful in induction therapy for
cryptococcal meningo-encephalitis.
Mode of action. Transported into fungal cells by a cytosine permease, host
cytosine deaminase converts 5-FC into 5-fluorouracil (5-FU). After
phosphorylation and incorporation into RNA, miscoding and disruption of
protein synthesis ensues. Also, phosphorylated 5-FU is converted to its
deoxynucleoside and blocks DNA synthesis by inhibiting thymidylate synthase.
Allylamines
Terbinafine is a lipophilic agent in the allylamine class of drugs.
Mode of action. Terbinafine inhibits an early step of ergosterol
biosynthesis, the squalene epoxidase enzyme, resulting in squalene
accumulation which increases membrane permeability and disruption of the
fungal cell.
Action spectrum. Terbinafine concentrates in the skin and nails after oral
administration and has a role in treating dermatophytosis including nail
infections. Topical formulations are sold over-the-counter. Terbinafine
combined with itraconazole or voriconazole may be synergistic in treating
particularly resistant melanized molds, such as Lomentospora (was
Scedosporium) prolificans. An explanation for this is that the two agents
block ergosterol at different points in its biosynthesis.
Echinocandins
This class of natural products has a cyclic hexapeptide structure linked to
an acyl lipid side chain. Anidulafungin discovered in 1974, is a
semi-synthetic modification of a product from Aspergillus nidulans and was
finally licensed in 2006. Caspofungin was approved in 2001 and micafungin in
2005. They are generally safe with few drug interactions. All three
echinocandins were developed for daily IV infusion.
Mode of action. Echinocandins are noncompetitive inhibitors of the plasma
membrane-bound β-(13)-D-glucan synthase enzyme complex, interfering with
synthesis of fibrillar β-(13)-D-glucan, a cell wall component of Candida
and of several other fungi. Without this glucan fungal cells become
osmotically fragile. Mammalian cells lack this polysaccharide so that
echinocandins have low host toxicity and reduced adverse effects.
Action spectrum. All three echinocandins are equally active as first line
therapy against candidemia and invasive candidiasis; they are cidal for
Candida spp. and fungistatic for molds. With respect to aspergillosis,
caspofungin is recommended only for salvage therapy. Echinocandins lack
activity against: Cryptococcus species, agents of the dimorphic endemic
mycoses, and the difficult to treat molds: Fusarium, Scedosporium, and
Mucorales.
Griseofulvin
A natural product of Penicllium griseofulvum, in 1952 it was recognized as
the “curling factor” producing distorted fungal hyphae. Dermatophytes were
very sensitive to griseofulvin but not yeast or bacteria. The key structural
feature of this unusual molecule is the spirobenzofuranone moiety. Oral
bioavailability is variable because it is poorly water-soluble.
Mode of Action. Griseofulvin inhibits mitosis strongly in fungal cells and
weakly in mammalian cells by affecting mitotic spindle microtubule function.
After oral administration, griseofulvin deposits in keratin precursor cells
with greater affinity for diseased tissue. The drug binds to new keratin
which becomes resistant to fungal invasion. Once the keratin-griseofulvin
complex reaches the skin site, it binds to fungal microtubules (tubulin)
altering fungal mitosis.
Action spectrum. Griseofulvin is a second line drug for the treatment of
ringworm of the skin, hair, and nails caused by Trichophyton or Microsporum
species. It is little used today because other drugs are more rapid acting
with greater efficacy. Adverse effects of griseofulvin are usually mild
including self-limited hepatotoxicity but very rare severe reactions are
known: toxic epidermal necrolysis and related Stevens-Johnson syndrome,
exacerbation of lupus.
Topical antifungal therapy
The preference for systemic or topical therapy of mucocutaneous and
cutaneous mycoses depends on the immune status of the host and the type and
extent of infection. Infections with dermatophytes, Candida spp. and
Malassezia spp. yeasts can be treated topically with a variety of
creams, lotions, ointments, powders, and spray forms. Terbinafine,
ketoconazole, or miconazole in creams or sprays are used for tinea pedis,
tinea cruris. Nystatin powder is used for intertriginous candidiasis and
ketoconazole cream for seborrheic dermatitis associated with Malassezia
spp. Oral clotrimazole troches, miconazole slow release tablets, or
nystatin suspension or pastilles are used to treat oropharyngeal
candidiasis. Vaginal candidiasis may be treated with creams or pessary form
of clotrimazole, or nystatin. Refractory dermatophytosis such as tinea
unguium and tinea capitis respond better to systemic therapy. Esophageal and
vulvovaginal candidiasis respond well to systemic fluconazole therapy.
|
|
|
 Return to the Mycology Section of Microbiology and Immunology On-line
Return to the Mycology Section of Microbiology and Immunology On-line
This page last changed on
Friday, December 28, 2018
Page maintained by
Richard Hunt
|

 Figure 1
Figure 1 Figure 4
Figure 4 Figure 8
Figure 8 Figure 9
Figure 9



