| x | x | |||||||||||||||||||||||||||||||||||||||||||||||||
 |
||||||||||||||||||||||||||||||||||||||||||||||||||
| IMMUNOLOGY | MYCOLOGY | PARASITOLOGY | VIROLOGY | |||||||||||||||||||||||||||||||||||||||||||||||
|
|
Dr Abdul
Ghaffar |
|||||||||||||||||||||||||||||||||||||||||||||||||
|
Let us know what you think |
||||||||||||||||||||||||||||||||||||||||||||||||||
|
|
||||||||||||||||||||||||||||||||||||||||||||||||||
| Logo image © Jeffrey Nelson, Rush University, Chicago, Illinois and The MicrobeLibrary | ||||||||||||||||||||||||||||||||||||||||||||||||||
|
|
||||||||||||||||||||||||||||||||||||||||||||||||||
|
TEACHING
OBJECTIVES
|
Hypersensitivity refers to excessive, undesirable (damaging, discomfort-producing and sometimes fatal) reactions produced by the normal immune system. Hypersensitivity reactions require a pre-sensitized (immune) state of the host. Hypersensitivity reactions can be divided into four types: type I, type II, type III and type IV, based on the mechanisms involved and time taken for the reaction. Frequently, a particular clinical condition (disease) may involve more than one type of reaction.
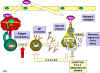
Type I Hypersensitivity Type I hypersensitivity is also known as immediate or anaphylactic hypersensitivity. The reaction may involve skin (urticaria and eczema), eyes (conjunctivitis), nasopharynx (rhinorrhea, rhinitis), bronchopulmonary tissues (asthma) and gastrointestinal tract (gastroenteritis). The reaction may cause a range of symptoms from minor inconvenience to death. The reaction usually takes 15 - 30 minutes from the time of exposure to the antigen, although sometimes it may have a delayed onset (10 - 12 hours). Immediate hypersensitivity is mediated by IgE. The primary cellular component in this hypersensitivity is the mast cell or basophil. The reaction is amplified and/or modified by platelets, neutrophils and eosinophils. A biopsy of the reaction site demonstrates mainly mast cells and eosinophils. The mechanism of reaction involves preferential production of IgE, in response to certain antigens (often called allergens). The precise mechanism as to why some individuals are more prone to type-I hypersensitivity is not clear. However, it has been shown that such individuals preferentially produce more of TH2 cells that secrete IL-4, IL-5 and IL-13 which in turn favor IgE class switch. IgE has very high affinity for its receptor (Fcε; CD23) on mast cells and basophils. A subsequent exposure to the same allergen cross links the cell-bound IgE and triggers the release of various pharmacologically active substances (figure 1). Cross-linking of IgE Fc-receptor is important in mast cell triggering. Mast cell degranulation is preceded by increased Ca++ influx, which is a crucial process; ionophores which increase cytoplasmic Ca++ also promote degranulation, whereas, agents which deplete cytoplasmic Ca++ suppress degranulation. The agents released from mast cells and their effects are listed in Table 1. Mast cells may be triggered by other stimuli such as exercise, emotional stress, chemicals (e.g., photographic developing medium, calcium ionophores, codeine, etc.), anaphylotoxins (e.g., C4a, C3a, C5a, etc.). These reactions, mediated by agents without IgE-allergen interaction, are not hypersensitivity reactions, although they produce the same symptoms.
|
|||||||||||||||||||||||||||||||||||||||||||||||||
|
The reaction is amplified by PAF
(platelet activation factor) which causes platelet aggregation and release of
histamine, heparin and vasoactive amines. Eosinophil chemotactic factor of
anaphylaxis (ECF-A) and neutrophil chemotactic factors attract eosinophils and
neutrophils, respectively, which release various hydrolytic enzymes that cause
necrosis. Eosinophils may also control the local reaction by releasing
arylsulphatase,
histaminase,
phospholipase-D and
prostaglandin-E, although this
role of eosinophils is now in question. |
||||||||||||||||||||||||||||||||||||||||||||||||||
|
Cyclic nucleotides appear to play a significant role in the modulation of immediate hypersensitivity reaction, although their exact function is ill understood. Substances which alter cAMP and cGMP levels significantly alter the allergic symptoms. Thus, substances that increase intracellular cAMP seem to relieve allergic symptoms, particularly broncho-pulmonary ones, and are used therapeutically (Table 2). Conversely, agents which decrease cAMP or stimulate cGMP aggravate these allergic conditions.
Diagnostic tests for immediate hypersensitivity include skin (prick and intradermal) tests (fig. 1A), measurement of total IgE and specific IgE antibodies against the suspected allergens. Total IgE and specific IgE antibodies are measured by a modification of enzyme immunoassay (ELISA). Increased IgE levels are indicative of an atopic condition, although IgE may be elevated in some non-atopic diseases (e.g., myelomas, helminthic infection, etc.). There appears to be a genetic predisposition for atopic diseases and there is evidence for HLA (A2) association. Symptomatic treatment is achieved with anti-histamines which block histamine receptors. Chromolyn sodium inhibits mast cell degranulation, probably, by inhibiting Ca++ influx. Late onset allergic symptoms, particularly bronchoconstriction which is mediated by leukotrienes, are treated with leukotriene receptor blockers (Singulair, Accolate) or inhibitors of the cyclooxygenase pathway (Zileutoin). Symptomatic, although short term, relief from bronchoconstriction is provided by bronchodilators (inhalants) such as isoproterenol derivatives (Terbutaline, Albuterol). Thophylline elevates cAMP by inhibiting cAMP-phosphodiesterase and inhibits intracellular Ca++ release is also used to relieve bronchopulmonary symptoms. The use of IgG antibodies against the Fc portions of IgE that binds to mast cells has been approved for treatment of certain allergies, as it can block mast cell sensitization. Hyposensitization
(immunotherapy or desensitization) is another treatment modality which is
successful in a number of allergies, particularly to insect venoms and, to some
extent, pollens. The mechanism is not clear, but there is a correlation between
appearance of IgG (blocking) antibodies and relief from symptoms. Suppressor T
cells that specifically inhibit IgE antibodies may play a role. |
||||||||||||||||||||||||||||||||||||||||||||||||||
 Figure 1A close-up view of intradermal skin test with multiple positive allergen responses
Figure 1A close-up view of intradermal skin test with multiple positive allergen responses © Bristol Biomedical Image Archive. Used with permission
|
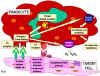
Type II Hypersensitivity Type II hypersensitivity is also known as cytotoxic hypersensitivity and may affect a variety of organs and tissues. The antigens are normally endogenous, although exogenous chemicals (haptens) which can attach to cell membranes can also lead to type II hypersensitivity. Drug-induced hemolytic anemia, granulocytopenia and thrombocytopenia are such examples. The reaction time is minutes to hours. Type II hypersensitivity is primarily mediated by antibodies of the IgM or IgG classes and complement (Figure 2). Phagocytes and K cells may also play a role. The lesion contains antibody, complement and neutrophils. Diagnostic tests include detection of circulating antibody against the tissues involved and the presence of antibody and complement in the lesion (biopsy) by immunofluorescence. The staining pattern is normally smooth and linear, such as that seen in Goodpasture's nephritis (renal and lung basement membrane) (figure 3A) and pemphigus (skin intercellular protein, desmosome) (figure 3B). Treatment involves anti-inflammatory and immunosuppressive agents.
Type III Hypersensitivity Type III hypersensitivity is also known as immune complex hypersensitivity. The reaction may be general (e.g., serum sickness) or may involve individual organs including skin (e.g., systemic lupus erythematosus, Arthus reaction), kidneys (e.g., lupus nephritis), lungs (e.g., aspergillosis), blood vessels (e.g., polyarteritis), joints (e.g., rheumatoid arthritis) or other organs. This reaction may be the pathogenic mechanism of diseases caused by many microorganisms. The reaction may take 3 - 10 hours after exposure to the antigen (as in Arthus reaction). It is mediated by soluble immune complexes. They are mostly of the IgG class, although IgM may also be involved. The antigen may be exogenous (chronic bacterial, viral or parasitic infections), or endogenous (non-organ specific autoimmunity: e.g., systemic lupus erythematosus, SLE). The antigen is soluble and not attached to the organ involved. Primary components are soluble immune complexes and complement (C3a, 4a and 5a). The damage is caused by platelets and neutrophils (Figure 4). The lesion contains primarily neutrophils and deposits of immune complexes and complement. Macrophages infiltrating in later stages may be involved in the healing process. The affinity of antibody and size of immune complexes are important in production of disease and determining the tissue involved. Diagnosis involves examination of tissue biopsies for deposits of immunoglobulin and complement by immunofluorescence microscopy. The immunofluorescent staining in type III hypersensitivity is granular (as opposed to linear in type II such as seen in Goodpasture's syndrome). The presence of immune complexes in serum and depletion in the level of complement are also diagnostic. Polyethylene glycol-mediated turbidity (nephelometry) binding of C1q and Raji cell test are utilized to detect immune complexes. Treatment includes anti-inflammatory agents.
|
|||||||||||||||||||||||||||||||||||||||||||||||||
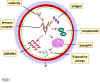 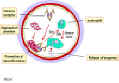
Figure 4. Mechanism of damage in immune complex hypersensitivity
|
Type IV Hypersensitivity Type IV hypersensitivity is also known as cell mediated or delayed type hypersensitivity. The classical example of this hypersensitivity is tuberculin (Montoux) reaction (figure 5) which peaks 48 hours after the injection of antigen (PPD or old tuberculin). The lesion is characterized by induration and erythema.
Type IV hypersensitivity is
involved in the pathogenesis of many autoimmune and infectious diseases
(tuberculosis, leprosy, blastomycosis, histoplasmosis, toxoplasmosis,
leishmaniasis, etc.) and
granulomas
due to infections and foreign
antigens. Another form of delayed hypersensitivity is contact dermatitis (poison
ivy (figure 6), chemicals, heavy metals, etc.) in which the lesions are more
papular.
Type IV hypersensitivity can be classified into three categories depending on
the time of onset and clinical and histological presentation (Table 3). |
|||||||||||||||||||||||||||||||||||||||||||||||||
 Figure 6 Poison Ivy CDC
Figure 6 Poison Ivy CDC |
Mechanisms of damage in delayed hypersensitivity include T lymphocytes and monocytes and/or macrophages. Cytotoxic T cells (Tc) cause direct damage whereas helper T (TH1) cells secrete cytokines which activate cytotoxic T cells and recruit and activate monocytes and macrophages, which cause the bulk of the damage (figure 4). The delayed hypersensitivity lesions mainly contain monocytes and a few T cells. Major lymphokines involved in delayed hypersensitivity reaction include monocyte chemotactic factor, interleukin-2, interferon-gamma, TNF alpha/beta, etc. Diagnostic tests in vivo include delayed cutaneous reaction (e.g. Montoux test (figure 5)) and patch test (for contact dermatitis). In vitro tests for delayed hypersensitivity include mitogenic response, lympho-cytotoxicity and IL-2 production. Corticosteroids and other immunosuppressive agents are used in treatment.
|
|||||||||||||||||||||||||||||||||||||||||||||||||
|
You have learned:
|
||||||||||||||||||||||||||||||||||||||||||||||||||
|
|
||||||||||||||||||||||||||||||||||||||||||||||||||