|
x |
x |
|
 |
 |
|
INFECTIOUS
DISEASE |
BACTERIOLOGY |
IMMUNOLOGY |
MYCOLOGY |
PARASITOLOGY |
VIROLOGY |
|
TURKISH |
BACTERIOLOGY - CHAPTER NINETEEN
MYCOPLASMA AND UREAPLASMA
Dr. Gene Mayer
Professor Emeritus
University of South Carolina School of Medicine
|
|
SPANISH |
|
SLOVAK |
|
ALBANIAN |
Let us know what you think
FEEDBACK |
|
SEARCH |
|
|
|
|
|
|
|
|

 |
|
Logo image © Jeffrey
Nelson, Rush University, Chicago, Illinois and
The MicrobeLibrary |
|
TEACHING OBJECTIVES
To describe the morphological and physiological
characteristics of the mycoplasmas
To discuss the pathogenesis of mycoplasma
infections
To describe the clinical syndromes associated with and
the epidemiology, diagnosis and treatment of mycoplasma infections
|
The family Mycoplasmataceae contains two genera that
infect humans: Mycoplasma and Ureaplasma, which are usually
referred to collectively as mycoplasmas. Although there are many species of
mycoplasmas, only four are recognized as human pathogens; Mycoplasma
pneumoniae, Mycoplasma hominis, Mycoplasma genitalium, and Ureaplasma
urealyticum. Although there are other species that have been isolated from
humans, their role in disease is not well established. The diseases caused by M.
pneumoniae, M. hominis, M. genitalium and U. urealyticum are
presented in Table 1 (Adapted from: Murray, et al., Medical Microbiology
3rd Ed., Table 42-1).
|
Table 1 |
|
Organism |
Disease |
|
M. pneumoniae |
Upper respiratory tract disease, tracheobronchitis,
atypical pneumonia |
|
M. hominis |
Pyelonephritis, pelvic inflammatory disease, postpartum
fever |
|
M. genitalium |
Non-gonococcal urethritis |
|
U. urealyticum |
Non-gonococcal urethritis |
|
|
KEY WORDS
T-strains
"Fried Egg"
Colonies
P1
Adhesin
Primary Atypical Pneumonia
Walking
Pneumonia
Cold Agglutinins
|
Morphology and Physiology
The mycoplasmas are the smallest free-living bacteria. They
range from 0.2 - 0.8 micrometers and thus can pass through some filters used to
remove bacteria. They have the smallest genome size and, as a result, lack many
metabolic pathways and require complex media for their isolation. The
mycoplasmas are facultative anaerobes, except for M. pneumoniae, which
is a strict aerobe. A characteristic feature that distinguishes the
mycoplasmas from other bacteria is the lack of a cell wall. Thus, they can
assume multiple shapes including round, pear shaped and even filamentous.
The mycoplasmas grow slowly by binary fission and produce
"fried egg" colonies on agar plates (Figure 1a); the colonies of M.
pneumoniae have a granular appearance. Due to the slow growth of
mycoplasmas, the colonies may take up to 3 weeks to develop and are usually
very small. The colonies of Ureaplasma are extremely small and thus Ureaplasma
are also called T-strains (tiny strains).
|
 Figure 1a
Figure 1a
Gram-negative Mycoplasma hominis, and T-strain
Mycoplasma isolates, which had been grown on agar medium. Members of the
genus Mycoplasma lack a cell wall, and are therefore, difficult to treat
with many antibiotics, which have a negative affect on bacterial
cell-wall synthesis such as penicillin. CDC
 Figure 1b
Transmission electron photomicrographs of the specialized tip organelle of
cytadherence-positive M. pneumoniae demonstrating a)
truncated structure with nap, b) clustering of cytadherence-related proteins (P1, B, C, P30) at the tip based on immunolabeling with ferritin and colloidal gold and crosslinking studies, and c) Triton X-100-resistant,
cytoskeleton-like, structure with distinct bleb and parallel filaments
Figure 1b
Transmission electron photomicrographs of the specialized tip organelle of
cytadherence-positive M. pneumoniae demonstrating a)
truncated structure with nap, b) clustering of cytadherence-related proteins (P1, B, C, P30) at the tip based on immunolabeling with ferritin and colloidal gold and crosslinking studies, and c) Triton X-100-resistant,
cytoskeleton-like, structure with distinct bleb and parallel filaments
 Transmission electron photomicrograph of a
Transmission electron photomicrograph of a
hamster trachea ring infected with M. pneumoniae. Note the orientation of the mycoplasmas through their
specialized tip-like organelle, which permits close association with the respiratory epithelium. M,
mycoplasma; m, microvillus; C, cilia.
Both images used with permission. From Baseman and Tully,
Emerging
Infection Diseases 3
|
The mycoplasma all require sterols for growth
and for membrane
synthesis. The three species can be differentiated by their ability to
metabolize glucose (M. pneumoniae), arginine (M. hominis) or
urea (U. urealyticum). The fourth species M. genitalium is
extremely difficult to culture.
Pathogenesis
Adherence factors
The mycoplasmas are extracellular
pathogens that adhere to epithelial cell surfaces. Thus, adherence proteins
are one of the major virulence factors. The adherence protein in M.
pneumoniae has been identified as a 168kD protein called P1. The P1
Adhesin localizes at tips of the bacterial cells and binds to sialic acid
residues on host epithelial cells (Figure 1b)
The nature of the adhesins in the other species has not been
established. Colonization of the respiratory tract by M. pneumoniae
results in the cessation of ciliary movement. The normal clearance mechanisms of
the respiratory tract do not function, resulting in contamination of the
respiratory tract and the development of a dry cough.
Toxic Metabolic Products
The intimate association of
the mycoplasma and the host cells provides an environment in which toxic
metabolic products accumulate and damage host tissues (Figure 1). Both
hydrogen peroxide and superoxide, which are products of mycoplasma
metabolism, have been implicated in pathogenesis since oxidized host lipids
have been found in infected tissues. Furthermore, the mycoplasmas have been
shown to inhibit host cell
catalase, thereby increasing the peroxide
concentrations.
Immunopathogenesis
Mycoplasmas can activate
macrophages and stimulate cytokine production and lymphocyte activation (M.
pneumoniae is a superantigen). Thus, it is has been suggested that host
factors also contribute to pathogenesis. Experimental evidence in animals
supports this suggestion. Ablation of thymus function before infection with M.
pneumoniae prevents the development of pneumonia and animals in which
thymic function is restored develop pneumonia at an exacerbated rate.
Epidemiologic data in humans suggest that repeated infections are required
before clinical disease is observed, again suggesting a role for host
related factors in pathogenesis; most children are infected from 2 - 5 years
of age but disease is most common in children 5-15 years of age.
|
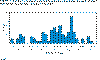 Acquired Pneumonia Caused by Mycoplasma pneumoniae
-- Colorado, 2000
Acquired Pneumonia Caused by Mycoplasma pneumoniae
-- Colorado, 2000
 Infections of children under 15 years of age in Seattle between 1969 and
1975. (From: Foy, J Infect Dis. 139, 681, 1979. Redrawn from : Murray, et al., Medical
Microbiology, 3rd Ed).
Infections of children under 15 years of age in Seattle between 1969 and
1975. (From: Foy, J Infect Dis. 139, 681, 1979. Redrawn from : Murray, et al., Medical
Microbiology, 3rd Ed).
Figure 2
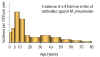 Antibody titers in different age groups. Anti-mycoplasma
pneumoniae antibodies indicate pneumonia caused by this organism is
highest in the 5-15 year age group (From: Foy, J Infect Dis. 139, 681, 1979. Redrawn from: Murray, et al., Medical
Microbiology, 3rd Ed).
Antibody titers in different age groups. Anti-mycoplasma
pneumoniae antibodies indicate pneumonia caused by this organism is
highest in the 5-15 year age group (From: Foy, J Infect Dis. 139, 681, 1979. Redrawn from: Murray, et al., Medical
Microbiology, 3rd Ed).
Figure 3
|
M. pneumoniae
Epidemiology
Pneumonia caused by M. pneumoniae
occurs worldwide and no increased seasonal activity is seen. However,
epidemics occur every 4 - 8 years (Figure 2).
The disease is spread by
close contact via aerosolized droplets and thus is most easily spread in
confined populations (e.g., families, schools, army barracks). The disease
is primarily one of the young (5 - 15 years of age - Figure 3)
Clinical syndrome
The most common clinical syndrome
following infection with M. pneumoniae is tracheobronchitis, which is
seen in 70-80% of the infections. Approximately one third of infected
persons will develop pneumonia which is usually mild but of long duration.
Pneumonia caused by this agent has been referred to a 'primary atypical
pneumonia' and 'walking pneumonia'. The clinical course of the
disease is depicted in Figure 4
The incubation time
following infection is approximately 2 - 3 weeks at which time fever,
headache and malaise are gradually observed. These symptoms may be
accompanied by a persistent non-productive hacking cough. Respiratory
symptoms appear somewhat later and persist for several weeks. Interestingly,
in M. pneumoniae pneumonia X-ray examination will show signs of
pneumonia even before respiratory symptoms appear. Organisms can be cultured
from sputum before symptoms occur and throughout the course of the disease.
Resolution of the disease is slow but it is rarely fatal. The disease must
be differentiated from other 'atypical' pneumonias.
Immunity
Complement activation via the alternative
pathway and phagocytic cells both play a role in resistance to infection. As
the infection proceeds, antibodies play a role in controlling infection,
particularly IgA. The development of delayed type hypersensitivity, however,
is associated with the severity of the disease, which supports the
suggestion that pathogenesis is at least, in part, immunopathogenesis.
Laboratory Diagnosis
In the early stages of
infection diagnosis must be made on clinical grounds. However, as the
infection progresses several laboratory tests are available.
-
Microscopy
This is not particularly useful because of
the absence of a cell wall but it can be helpful in eliminating other possible
pathogens.
-
Culture
Sputum (usually scant) or throat
washings must be sent to the laboratory in special transport medium. It may
take 2 -3 weeks to get a positive identification. Culture is essential
for a definitive diagnosis.
|
|
WEB RESOURCES
Mycoplasmas: Sophisticated, Reemerging, and Burdened by Their Notoriety
From Emerging Infectious Diseases |
 Figure 4
Figure 4 |
-
Complement fixation test - There is a good
complement fixation test that has good sensitivity and specificity.
However, the titers do not peak until 4 - 6 weeks after infection
(Figure 4). A fourfold rise in titer is indicative of a recent
infection. Since antibodies may persist for up to 1 year, a sustained
high titer does not necessarily indicate a current infection.
-
Cold agglutinins - Approximately 34% - 68% of
patients with M. pneumoniae infection develop cold agglutinins.
Cold agglutinins are antibodies that agglutinate human erythrocytes at
4 degrees C but not at 37 degrees C. The antigen to which the
antibodies are directed is the I antigen. These antibodies arise
before the complement fixing antibodies and they decline faster
(Figure 4). Cold agglutinins are not specific for M. pneumoniae
infections, they can also appear in other infections and in other
diseases (e.g. Infectious mononucleosis, influenza infections,
cold agglutinin disease, leukemia). However, if present in a patient
with clinical signs of M. pneumoniae infection, a presumptive
diagnosis can be made.
-
ELISA - There is a new ELISA for IgM that has
been used for diagnosis of acute infection. It is sensitive and
specific. However, it is not yet commercially available.
Treatment and Prevention
Since mycoplasmas
lack a cell wall, the penicillins and cephalosporins are ineffective. The
antibiotics of choice are tetracycline (adults only) and erythromycin.
Prevention is a problem due to the long duration of the disease. It is
problematic to isolate patients to avoid close contact for a long period of
time. No vaccines are currently available.
|
| |
M. hominis and U. urealyticum
Clinical syndromes
M. hominis is associated
with
pyelonephritis, pelvic inflammatory disease and
post-partum fevers. U.
urealyticum is associated with non-gonococcal urethritis.
Epidemiology
Colonization with M. hominis and
U. urealyticum can occur during birth but in most cases the infection
will be cleared. Only in a small number of cases does colonization persist.
However, when individuals become sexually active, colonization rates
increase. Approximately 15% are colonized with M. hominis and 45% -
75% with U. urealyticum. The carriers are asymptomatic but the
organisms can be opportunistic pathogens.
Laboratory Diagnosis
Laboratory diagnosis is by
culture.
Treatment and Prevention
Since mycoplasmas
lack a cell wall, the penicillins and cephalosporins are ineffective. The
antibiotics of choice are tetracycline (adults only) and erythromycin.
Abstinence or proper barrier protection are means of prevention.
|
|
|
 Return to the Bacteriology Section
of Microbiology and Immunology On-line Return to the Bacteriology Section
of Microbiology and Immunology On-line
This page last changed on
Sunday, March 06, 2016
Page maintained by
Richard Hunt
|

 Figure 1a
Figure 1a Acquired Pneumonia Caused by Mycoplasma pneumoniae
-- Colorado, 2000
Acquired Pneumonia Caused by Mycoplasma pneumoniae
-- Colorado, 2000
 Figure 4
Figure 4