|
x |
x |
|
 |
 |
|
INFECTIOUS
DISEASE |
BACTERIOLOGY |
IMMUNOLOGY |
MYCOLOGY |
PARASITOLOGY |
VIROLOGY |
|
VIETNAMESE |
IMMUNOLOGY - CHAPTER SEVEN
IMMUNOGLOBULINS- ANTIGEN-ANTIBODY REACTIONS
AND SELECTED TESTS
Gene Mayer, Ph.D
Emertius Professor of Pathology, Microbiology and Immunology
University of South Carolina
|
|
TURKISH |
|
FRANCAIS |
|
PORTUGUES |
|
SHQIP |
|
Let us know what you think
FEEDBACK |
|
SEARCH |
|
|
|

 |
|
Logo image
© Jeffrey Nelson, Rush University, Chicago, Illinois and
The MicrobeLibrary |
|
|
|
|
|
TEACHING
OBJECTIVES
To
describe the nature of Ag-Ab reactions
To
compare and contrast antibody affinity and avidity
To
delineate the basis for antibody specificity and cross reactivity
To
discuss the principles of commonly used tests for antigen/antibody
reactions
 Figure 1
Figure 1 |
NATURE OF ANTIGEN-ANTIBODY REACTIONS
Lock and Key Concept
The combining site of an antibody is located in the Fab portion of the
molecule and is constructed from the
hypervariable regions of the heavy
and light chains. X-Ray crystallography studies of antigen-antibody
interactions show that the antigenic determinant nestles in a cleft
formed by the combining site of the antibody as illustrated in Figure 1.
Thus, our concept of antigen-antibody reactions is one of a key (i.e. the
antigen) which
fits into a lock (i.e. the antibody).
Non-covalent Bonds
The bonds that hold the antigen to the antibody combining site are all
non-covalent in nature. These include
hydrogen bonds,
electrostatic bonds,
Van der Waals forces and
hydrophobic bonds. Multiple bonding between the
antigen and the antibody ensures that the antigen will be bound tightly to the
antibody.
Reversibility
Since antigen-antibody
reactions occur via non-covalent bonds, they are by their nature
reversible.
|
|
KEY WORDS
Affinity
Avidity
Specificity
Cross
reactivity
Agglutination
Hemagglutination
Agglutinin
Titer
Prozone
Passive hemagglutination
Direct
Coomb's test
Indirect
Coomb's test
Hemagglutination
inhibition
Equivalence
point
Antibody
excess
Antigen excess
Radial
immunodiffusion
Immunoelectrophoresis
Countercurrent
immunoelectrophoresis
Radioimmunoassay
Enzyme linked
immunosorbent assay
Competitive RIA/ELISA
Noncompetitive RIA/ELISA
Immunofluorescence
Flow
cytometry
Complement fixation
 Figure
2
Figure
2
 Figure
3
Figure
3
 Figure
4
Figure
4
 Figure
5
Figure
5 |
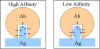
AFFINITY AND AVIDITY
Affinity
Antibody
affinity is the strength of the reaction between a single antigenic
determinant and a single combining site on the antibody. It is the sum of
the attractive and repulsive forces operating between the antigenic
determinant and the combining site of the antibody as illustrated in
Figure 2.
Affinity is the equilibrium
constant that describes the antigen-antibody reaction as illustrated in Figure 3.
Most antibodies have a high affinity for their antigens.
Avidity
Avidity is a
measure of the overall strength of binding of an antigen with many
antigenic determinants and multivalent antibodies. Avidity is
influenced by both the valence of the antibody and the valence of the
antigen. Avidity is more than the sum of the individual affinities. This
is illustrated in Figure 4.
To repeat, affinity refers to
the strength of binding between a single antigenic determinant and an
individual antibody combining site whereas avidity refers to the overall
strength of binding between multivalent antigens and antibodies.
SPECIFICITY AND CROSS
REACTIVITY
Specificity
Specificity refers to the ability of an individual antibody combining
site to react with only one antigenic determinant or the ability of a
population of antibody molecules to react with only one antigen. In
general, there is a high degree of specificity in antigen-antibody reactions.
Antibodies can distinguish differences in:
-
The primary structure of an
antigen
-
Isomeric forms of an antigen
-
Secondary and tertiary
structure of an antigen
Cross reactivity
Cross reactivity refers to the ability of an individual antibody combining
site to react with more than one antigenic determinant or the ability of a
population of antibody molecules to react with more than one antigen.
Figure 5 illustrates how cross reactions can arise. Cross reactions arise
because the cross reacting antigen shares an
epitope in common with the
immunizing antigen or because it has an epitope which is structurally
similar to one on the immunizing antigen (multispecificity).
TESTS FOR ANTIGEN-ANTIBODY
REACTIONS
Factors affecting
measurement of antigen-antibody reactions
The only way that one knows that an
antigen-antibody reaction has occurred is to have some means of directly
or indirectly detecting the complexes formed between the antigen and
antibody. The ease with which one can detect antigen-antibody reactions
will depend on a number of factors.
Affinity
The higher the
affinity of the antibody for the antigen, the more stable will be the
interaction. Thus, the ease with which one can detect the interaction is
enhanced.
Avidity
Reactions between
multivalent antigens and multivalent antibodies are more stable and thus
easier to detect.
|
 Figure
6
Figure
6 |
Antigen to antibody ratio
The ratio between the antigen and antibody influences the detection
of antigen-antibody complexes because the size of the complexes formed is related to the
concentration of the antigen and antibody. This is depicted in Figure 6.
Physical form of the antigen
The physical form of the antigen influences how one detects its reaction
with an antibody. If the antigen is a particulate, one generally looks
for agglutination of the antigen by the antibody. If the antigen is
soluble one generally looks for the precipitation of the antigen after
the production of large insoluble antigen-antibody complexes.
|
 Figure 7
Figure 7 |
Agglutination Tests
Agglutination/Hemagglutination
When the antigen is particulate, the reaction of an antibody with the
antigen can be detected by agglutination (clumping) of the antigen. The
general term agglutinin is used to describe antibodies that agglutinate
particulate antigens. When
the antigen is an erythrocyte the term
hemagglutination
is used. All antibodies can theoretically agglutinate particulate
antigens but IgM, due to its high valence, is particularly good agglutinin
and one sometimes infers that an antibody may be of the IgM class if it is
a good agglutinating antibody.
Qualitative agglutination
test
Agglutination tests can be used in a
qualitative manner to assay for the presence of an antigen or an
antibody. The antibody is mixed with the particulate antigen and a
positive test is indicated by the agglutination of the particulate
antigen. (Figure 7).
For example, a patient's red blood cells
can be mixed with antibody to a blood group
antigen to determine a person's blood type. In a second example, a
patient's serum is mixed with red blood cells of a known blood type to
assay for the presence of antibodies to that blood type in the
patient's serum.
|
 Figure 8
Figure 8 |
Quantitative agglutination
test
Agglutination tests can also be used to
measure the level of antibodies to particulate antigens. In this test, serial dilutions
are made of a sample to be tested for antibody and
then a fixed number of red blood cells or bacteria or other such
particulate antigen is added. Then the maximum dilution that gives
agglutination is determined. The maximum dilution that gives visible agglutination is
called the
titer. The results are reported as the reciprocal of
the maximal dilution that gives visible agglutination. Figure 8
illustrates a quantitative hemagglutination test.
Prozone effect - Occasionally,
it is observed that when the concentration of antibody is high (i.e. lower
dilutions), there is no agglutination and then, as the sample is diluted,
agglutination occurs (See Patient 6 in Figure 8). The lack of
agglutination at high concentrations of antibodies is called the
prozone
effect. Lack of agglutination in the prozone is due to antibody excess
resulting in very small complexes that do not clump to form visible
agglutination.
|
| |
Applications of
agglutination tests
i. Determination of blood
types or antibodies to blood group antigens.
ii. To assess bacterial
infections
e.g.
A rise in titer of an antibody to a particular bacterium indicates an infection with
that bacterial type. N.B. a fourfold rise in titer is generally taken as a
significant rise in antibody titer.
Practical considerations
Although the test is easy to perform, it is only semi-quantitative.
|
 Figure
9
Figure
9 |
Passive hemagglutination
The agglutination test only works with particulate antigens. However, it
is possible to coat erythrocytes with a soluble antigen (e.g. viral
antigen, a polysaccharide or a hapten) and use the coated red blood cells
in an agglutination test for antibody to the soluble antigen (Figure 9).
This is called passive hemagglutination. The test is performed just like
the agglutination test. Applications include detection of antibodies to
soluble antigens and detection of antibodies to viral antigens.
|
 Figure
10
Figure
10 |
Coomb's Test (Antiglobulin
Test)
Direct Coomb's Test
When antibodies bind to erythrocytes, they do not always result in
agglutination. This can result from the antigen/antibody ratio being in antigen
excess or antibody excess or in some cases electrical charges on the red
blood cells preventing the effective cross linking of the cells. These
antibodies that bind to but do not cause agglutination of red blood
cells are sometimes referred to as incomplete antibodies. In no way is
this meant to indicate that the antibodies are different in their
structure, although this was once thought to be the case. Rather, it is
a functional definition only. In order to detect the presence of
non-agglutinating antibodies on red blood cells, one simply adds a
second antibody directed against the immunoglobulin (antibody) coating the red
cells. This anti-immunoglobulin can now cross link the red blood cells
and result in agglutination. This test is illustrated in Figure 10 and
is known as the
Direct Coomb's test.
|
 Figure
11
Figure
11 |
Indirect Coomb's Test
If it is necessary to know whether a serum sample has antibodies
directed against a particular red blood cell and you want to be sure
that you also detect potential non- agglutinating antibodies in the
sample, an
Indirect Coomb's test is performed (Figure 11). This test is
done by incubating the red blood cells with the serum sample, washing
out any unbound antibodies and then adding a second anti-immunoglobulin
reagent to cross link the cells.
Applications
These
include detection of anti-rhesus
factor (Rh) antibodies. Antibodies to the Rh factor
generally do not agglutinate red blood cells. Thus, red cells from Rh+
children born to Rh- mothers, who have anti-Rh antibodies,
may be coated with these antibodies. To check for this, a direct Coombs
test is performed. To see if the mother has anti-Rh antibodies in her
serum an Indirect Coombs test is performed.
|
 Figure
12
Figure
12 |
Hemagglutination Inhibition
The agglutination test can be modified to be used for the
measurement of soluble antigens. This test is called hemagglutination
inhibition. It is called hemagglutination inhibition because one measures
the ability of soluble antigen to inhibit the agglutination of
antigen-coated red blood cells by antibodies. In this test, a fixed amount
of antibodies to the antigen in question is mixed with a fixed amount of
red blood cells coated with the antigen (see passive hemagglutination
above). Also included in the mixture are different amounts of the sample
to be analyzed for the presence of the antigen. If the sample contains the
antigen, the soluble antigen will compete with the antigen coated on the
red blood cells for binding to the antibodies, thereby inhibiting the agglutination of
the red blood cells. as illustrated in Figure 12.
By serially diluting the sample,
you can quantitate the amount of antigen in your unknown sample by its
titer. This test is generally used to quantitate soluble antigens and is
subject to the same practical considerations as the agglutination test.
|
 Figure 13
Figure 13 |
Precipitation tests
Radial Immunodiffusion
(Mancini)
In radial immunodiffusion antibody is incorporated into
the agar gel as it is poured and different dilutions of the antigen are
placed in holes punched into the agar. As the antigen diffuses into the
gel, it reacts with the antibody and when the equivalence point is reached
a ring of precipitation is formed as illustrated in Figure 13.
The diameter of the ring is
proportional to the log of the concentration of antigen since the amount
of antibody is constant. Thus, by running different concentrations of a
standard antigen one can generate a standard cure from which one can
quantitate the amount of an antigen in an unknown sample. Thus, this is a
quantitative test. If more than one ring appears in the test, more than
one antigen/antibody reaction has occurred. This could be due to a mixture
of antigens or antibodies. This test is commonly used in the clinical
laboratory for the determination of immunoglobulin levels in patient
samples.
|
 Figure
14
Figure
14 |
Immunoelectrophoresis
In immunoelectrophoresis, a complex mixture of antigens is placed in a well
punched out of an agar gel and the antigens are electrophoresed so that
the antigen are separated according to their charge. After electrophoresis,
a trough is cut in the gel and antibodies are added. As the antibodies
diffuse into the agar, precipitin lines are produced in the equivalence
zone when an antigen/antibody reaction occurs as illustrated in Figure 14.
This tests is used for the
qualitative analysis of complex mixtures of antigens, although a crude
measure of quantity (thickness of the line) can be obtained. This test is
commonly used for the analysis of components in a patient' serum. Serum is
placed in the well and antibody to whole serum in the trough. By
comparisons to normal serum, one can determine whether there are
deficiencies on one or more serum components or whether there is an
overabundance of some serum component (thickness of the line). This test
can also be used to evaluate purity of isolated serum proteins.
|
 Figure
15
Figure
15 |
Countercurrent
electrophoresis
In this test the antigen and antibody are placed in
wells punched out of an agar gel and the antigen and antibody are electrophoresed into each other where they form a precipitation line as
illustrated in Figure 15. This test only works if conditions can be found
where the antigen and antibody have opposite charges. This test is
primarily qualitative, although from the thickness of the band you can get
some measure of quantity. Its major advantage is its speed.
|
 Figure
16
Figure
16
 Figure
17
Figure
17 |
Radioimmunoassay (RIA)/Enzyme
Linked Immunosorbent Assay (ELISA)
Radioimmunoassays (RIA) are assays
that are based on the measurement of radioactivity associated with immune
complexes. In any particular test, the label may be on either the antigen or
the antibody. Enzyme Linked Immunosorbent Assays (ELISA) are those that are
based on the measurement of an enzymatic reaction associated with immune
complexes. In any particular assay, the enzyme may be linked to either the
antigen or the antibody.
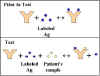
Competitive RIA/ELISA for Ag Detection
The method and principle of RIA and ELISA for the
measurement of antigen is shown in Figure 16. By using known amounts of a
standard unlabeled antigen, one can generate a standard curve relating
radioactivity (cpm)
(Enzyme) bound versus amount of antigen. From this standard curve, one can
determine the amount of an antigen in an unknown sample.
The key to the assay is the
separation of the immune complexes from the remainder of the components.
This has been accomplished in many different ways and serves as the basis
for the names given to the assay:
Precipitation with
ammonium sulphate
Ammonium sulphate (33 - 50% final concentration)
will precipitate immunoglobulins but not many antigens. Thus, this
can be used to separate the immune complexes from free antigen. This
has been called the Farr Technique
Anti-immunoglobulin
antibody
The addition of a second antibody directed against the
first antibody can result in the precipitation of the immune
complexes and thus the separation of the complexes from free
antigen.
Immobilization of the
Antibody
The antibody can be immobilized onto the surface of a
plastic bead or coated onto the surface of a plastic plate and thus
the immune complexes can easily be separated from the other
components by simply washing the beads or plate (Figure 17). This is
the most common method used today and is referred to as Solid phase RIA or ELISA. In the clinical laboratory, competitive RIA and ELISA
are commonly used to quantitate serum proteins, hormones, drugs
metabolites.
|
|
TUTORIAL
ELIZA ASSAY
HHMI
Requires Flash |
 Figure
18
Figure
18
 Figure
19
Figure
19 |
Non-competitive RIA/ELISA
for Ag or Ab
Non-competitive RIA and ELISAs are also used for the
measurement of antigens and antibodies. In Figure 18, the bead is coated
with the antigen and is used for the detection of antibody in the unknown
sample. The amount of labeled second antibody bound is related to the
amount of antibody in the unknown sample. This assay is commonly employed
for the measurement of antibodies of the IgE class directed against
particular allergens by using a known allergen as antigen and anti-IgE
antibodies as the labeled reagent. It is called the RAST test (radioallergosorbent
test). In Figure 19, the bead is coated with antibody and is used to
measure an unknown antigen. The amount of labeled second antibody that
binds is proportional to the amount of antigen that bound to the first
antibody.
|
 Figure
20
Figure
20 |
Tests for Cell Associated
Antigens
Immunofluorescence
Immunofluorescence is a technique whereby an antibody labeled with a
fluorescent molecule (fluorescein or rhodamine or one of many other
fluorescent dyes) is used to detect the
presence of an antigen in or on a cell or tissue by the fluorescence
emitted by the bound antibody.
Direct Immunofluorescence
In direct immunofluorescence, the antibody
specific to the antigen is directly tagged with the
fluorochrome (Figure
20).
|
 Figure
21
Figure
21 |
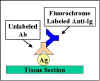
Indirect
Immunofluorescence
In indirect immunofluorescence, the antibody
specific for the antigen is unlabeled and a second anti-immunoglobulin
antibody directed toward the first antibody is tagged with the
fluorochrome (Figure 21). Indirect fluorescence is more sensitive than
direct immunofluorescence since there is amplification of the signal.
|
 Figure
22
Figure
22 |
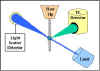
Flow Cytometry
Flow cytometry is commonly used in the clinical laboratory to identify
and enumerate cells bearing a particular antigen. Cells in suspension
are labeled with a fluorescent tag by either direct or indirect
immunofluorescence. The cells are then analyzed on the flow cytometer.
Figure 22 illustrates the
principle of flow cytometry. In a flow cytometer, the cells exit a flow
cell and are illuminated with a laser beam. The amount of laser light
that is scattered off the cells as they passes through the laser can be
measured, which gives information concerning the size of the cells. In
addition, the laser can excite the fluorochrome on the cells and the
fluorescent light emitted by the cells can be measured by one or more
detectors.
|
 Figure
23
Figure
23 |
The type of data that is
obtained from the flow cytometer is shown in Figure 23. In a one
parameter histogram, increasing amount of fluorescence (e.g.
green fluorescence) is plotted on the x axis and the number of cells
exhibiting that amount of fluorescence is plotted on the y axis. The
fraction of cells that are fluorescent can be determined by integrating
the area under the curve. In a two parameter histogram, the x axis is one
parameter (e.g. red fluorescence) and the y axis is the second
parameter (e.g. green fluorescence). The number of cells is
indicated by the contour and the intensity of the color.
|
 Figure
24
Figure
24
 PowerPoint animation of figure 24 of this figure
PowerPoint animation of figure 24 of this figure |
Complement Fixation
Antigen/antibody complexes can also be measured by their ability to fix
complement because an antigen/antibody complex will "consume" complement if
it is present, whereas free antigens or antibodies do not. Tests for antigen/antibody
complexes that rely on the consumption of complement are termed
complement fixation tests and are used to quantitate antigen/antibody reactions. This test will only work
with complement fixing antibodies (IgG and IgM are best).
The principle of the complement
fixation test is illustrated in Figure 24. Antigen is mixed with the
test serum to be assayed for antibody and antigen/antibody complexes are
allowed to form. A control tube in which no antigen is added is also
prepared. If no antigen/antibody complexes are present in the tube, none
of the complement will be fixed. However, if antigen/antibody complexes
are present, they will fix complement and thereby reduce the amount of
complement in the tube. After allowing complement fixation by any antigen/antibody complexes, a standard amount of red blood
cells, which have been pre-coated with anti-erythrocyte antibodies is
added. The amount of antibody-coated red blood cells is predetermined to be just enough
to completely use up all the complement initially added, if it were still
there. If all the complement was still present (i.e. no antigen/antibody
complexes formed between the antigen and antibody in question), all the
red cells will be lysed. If antigen/antibody complexes are formed
between the antigen and antibody in question, some of the
complement will be consumed and, thus, when the antibody-coated red cells are
added not all of them will lyse. By simply measuring the amount of red
cell lysis by measuring the release of hemoglobin into the medium, one
can indirectly quantitate antigen/antibody complexes in the tube. Complement fixation
tests are most commonly used to assay for antibody in a test sample but
they can be modified to measure antigen.
|
| |
 Return to the Immunology Section of Microbiology and Immunology
On-line
Return to the Immunology Section of Microbiology and Immunology
On-line
|
|


|
This page last changed on
Thursday, September 14, 2017
Page maintained by
Richard Hunt
|

 Figure
6
Figure
6 Figure 8
Figure 8 Figure
12
Figure
12 Figure 13
Figure 13 Figure
16
Figure
16
 Figure
20
Figure
20 Figure
21
Figure
21 Figure
22
Figure
22 Figure
24
Figure
24




