|
|
Common
characteristics
- These are the common characteristics of endemic dimorphic
mycoses:
They cause community acquired infections and are capable of causing
systemic disease in immune-normal and immunocompromised humans and
animals
- The mold forms occur in soil or plant matter in certain
geographic areas
- Each etiologic agent is morphologically distinct.
- They all exhibit dimorphism: def: existence is in two distinct
forms:
- The environmental form is as soil-dwelling mold.
- In the host the conidia germinate and undergo
temperature-sensitive conversion to the tissue form as either
yeast forms, fission yeast, or endosporulating spherules.
- Transmission is via inhalation of conidia (spores) from the
environment
- They are not communicable among people (with rare
exceptions).
- Inhalation of conidia initiates a pulmonary infection.
- The conidia germinate and convert to the tissue form (see
above: dimorphism)
- Depending on the inhaled dose, immune, and endocrine status
of the host: fungi may then either be phagocytosed, walled off
in granulomas, or are killed (most patients), or go on to
produce pneumonia and, in a small fraction of patients,
disseminate to other organs, including skin.
- Host response is T-cell mediated, isolating the fungi in
granulomas. i.e.: fungi are surrounded by macrophages
which may combine to form multinucleate giant cells
- Subclinical exposure resulting in self-limited infection
- Community acquired pneumonia
- Chronic lung disease
- Extra pulmonary dissemination
List of the Endemic Dimorphic Mycoses
Blastomycosis
Coccidioidomycosis
Histoplasmosis
Paracoccidioidomycosis
(the above fungi are phylogenetically related within the family
Onygenaceae)
Sporotrichosis
Talaromycosis (formerly Penicilliosis)
BLASTOMYCOSIS (Blastomyces
dermatitidis)
Disease Definition
Blastomycosis is a slowly progressing chronic pyo-granulomatous
disease of humans and dogs, most often presenting in a pulmonary
and/or cutaneous clinical form. The respiratory route is the most
important for infection via inhaling conidia or mycelial elements
from aerosolized soil, or from vegetative material.
- Blastomycosis is a rural disease but isolation of the
causative agent from the environment is uncommon.
- The patient presents with respiratory symptoms, loss of
appetite, weight loss, fever, productive cough, and night
sweats.
- Symptomatic disease may be present in less than half of
infected persons; others may have a "flu-like" response to
infection.
- Cutaneous lesions are most often secondary to hematogenous
spread. Primary cutaneous blastomycosis can occur but is
uncommon (see below Clinical Forms).
- Pulmonary and skin (fig 1) involvement are most common, but
bone, prostate are other sites of dissemination also including
other organs.
- Blastomycosis is correctly suspected in only a small
percentage of patients at the first clinical evaluation.
- The differential diagnosis includes bacterial pneumonia,
cancer, or tuberculosis.
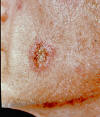 Fig. 1. Skin lesion, face, blastomycosis. This 54 y.o. man was seen
in the early 1960’s. He worked in a print shop in an Atlanta suburb.
It is unlikely that he traveled out of state. He had pulmonary
blastomycosis about 2 or 3 y before the current admission. When skin
lesions appeared he was referred to the Medical College of Georgia,
where he received a course of Amphotericin B.
Fig. 1. Skin lesion, face, blastomycosis. This 54 y.o. man was seen
in the early 1960’s. He worked in a print shop in an Atlanta suburb.
It is unlikely that he traveled out of state. He had pulmonary
blastomycosis about 2 or 3 y before the current admission. When skin
lesions appeared he was referred to the Medical College of Georgia,
where he received a course of Amphotericin B.
Photo credit: Dr.
Arthur F. DiSalvo
Etiologic agent
Blastomyces dermatitidis is a dimorphic fungus existing as a mold
form in soil or vegetative debris and, following inhalation of the
conidia or mycelial elements, changes to a monopolar budding yeast
form. The yeast form may also be demonstrated in the lab by
cultivation at 37oC.
Two evolutionary independent lineages of Blastomyces species were
discovered by applying multilocus sequence typing using 7 nuclear
loci. A genetically divergent clade within B. dermatitidis was
described as a new species, B. gilchristii (Brown et al., 2013).
Differences in geographic distribution and virulence are discussed
in Geographic Distribution (below).
Diagnosis
To make the specific diagnosis, the physician must be
aware of blastomycosis. Sputum sent to the lab for "culture" may not
grow unless the lab is alerted to look for fungi, generally, or
specifically for Blastomyces. In that case the lab will use fungal
media for isolation (see Laboratory, below). A typical cutaneous
lesion shows central healing with microabscesses at the periphery.
B. dermatitidis yeast forms can frequently be demonstrated in a KOH
prep of pus from such a lesion.
Risk factors
Blastomycosis is most often a rural disease. Blastomyces spp.
infect immune-normal as well as immunocompromised people who become
infected because, through recreation or occupation, they disturb the
environment: collecting firewood, tearing down old buildings.
Geographic Distribution and
Ecologic Niche
Blastomycosis occurs in eastern North America (fig 2). It is endemic
in southern and SE states that border the Ohio River and Mississippi
River valleys of the U.S., the midwestern states, and Canadian
provinces bordering the Great Lakes and the Saint Lawrence River.
Most reported cases occurred in Arkansas, Kentucky, Mississippi,
North Carolina, Tennessee, Louisiana, Illinois, and Wisconsin. The
disease is hyperendemic in north-central Wisconsin and the northern
region of Ontario, Canada.
The ecologic niche where the sexual reproduction, growth, and
dispersal of B. dermatitidis and B. gilchristii occur is linked to
freshwater systems.
B. dermatitidis isolates were recovered from human patients
and canines in areas throughout the endemic region in North America,
whereas B. gilchristii strains are restricted to Canada and some
northern U.S. states. Both species are associated with major
freshwater drainage basins.
B. dermatitidis populations are found in the:
- Nelson River drainage basin
- St. Lawrence River and northeast Atlantic Ocean Seaboard
drainage basins
- Mississippi River System drainage basin
- Gulf of Mexico Seaboard and southeast Atlantic Ocean
Seaboard drainage basins.
B. gilchristii populations occur among the more northerly
drainage basins only.
 Fig. 2. Areas endemic for blastomycosis in the United States
extending into Canada.
Fig. 2. Areas endemic for blastomycosis in the United States
extending into Canada.
Source: CDC
Blastomycosis outside
the U.S.
Authentic cases of blastomycosis also occur in Africa,
specifically South Africa and Zimbabwe. Infections have also been
reported in India. Such reports are to be viewed with caution
because physicians, unfamiliar with the disease, may invoke a wrong
diagnosis. B. dermatitidis can be transferred via fomites
from a known endemic area to another area where the disease may be
recognized. Blastomycosis may be recur because of endogenous
reactivation after a person has relocated.
Ecologic niche
The ecologic niche of B. dermatitidis is wet soil containing
animal droppings, rotting wood, other decaying vegetable matter.
Disruption of these environments containing microfoci of B. dermatitidis mycelia releases infectious conidia, which may be
inhaled by a susceptible host.
Clinical Forms
Pulmonary blastomycosis is seen in four broad categories:
• Asymptomatic, with only serologic evidence of prior infection or
granulomas
• Acute localized pneumonia
• Severe acute respiratory distress syndrome (ARDS)
• Subacute to chronic infiltrates and/or cavitary
disease
Chest X-ray (fig 3) shows obvious pulmonary disease.
Primary cutaneous blastomycosis caused by traumatic inoculation with
the organism, is uncommon, with fewer than 50 reported cases (Ladizinski
et al 2018) e.g.: among laboratory or morgue workers, dog handlers
after a bite or scratch, tree bark trauma, sawhorse-related injury,
grain elevator door–related trauma.
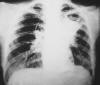 Fig. 3. Blastomycosis: Chest X-ray demonstrates lung infiltrates due
to blastomycosis.
Fig. 3. Blastomycosis: Chest X-ray demonstrates lung infiltrates due
to blastomycosis.
Source: CDC Public Health Image Library #5801 Dr.
Hardin
Laboratory
If there are skin lesions, send skin scrapings or pus. If there is
pulmonary involvement, send sputum or bronchial washings. Other
specimens include biopsy material and urine. Occasionally, the
organism can be isolated from urine as it often infects the
prostate. A pus specimen from a skin lesion may be obtained by
nicking the top of a microabscess with a scalpel, obtaining the
purulent material and making a KOH prep for microscopic exam. The
yeast form has a characteristic double contoured wall with a single
bud on a wide base (figs 4 - 5).
Specimens should be seeded to SDA or inhibitory mold agar. Addition
of cycloheximide and chloramphenicol will inhibit bacteria and
rapid-growing fungal saprobes. Media with and without antibiotics
are preferred. For tissue specimens, an enriched medium like brain
heart infusion + 5% sheep RBC and antibiotics is recommended.
After planting the specimen to an agar slant or plate, incubation is
conducted at both 37 o C and at 25 o C because, B. dermatitidis is
dimorphic. Culture of B. dermatitidis takes 2 to 3 wks to
grow at 25 oC.
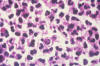 Fig. 4. Histopathology of a blastomycosis skin lesion. Budding yeast
of Blastomyces dermatitidis surrounded by neutrophils. Multiple
nuclei are visible in the yeast form.
Fig. 4. Histopathology of a blastomycosis skin lesion. Budding yeast
of Blastomyces dermatitidis surrounded by neutrophils. Multiple
nuclei are visible in the yeast form.
Source: Dr. Edwin P. Ewing,
Jr., CDC Public Health Image Library (PHIL) #491.
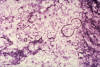 Fig. 5. Photomicrograph of a smear specimen from a foot lesion in a
case of blastomycosis. B. dermatitidis yeast cell is undergoing
broad-base budding.
Fig. 5. Photomicrograph of a smear specimen from a foot lesion in a
case of blastomycosis. B. dermatitidis yeast cell is undergoing
broad-base budding.
Source: #489 CDC PHIL
Colony morphology
A white, cottony mycelium on Sabouraud -dextrose agar.
Microscopic morphology. The conidia, are evident but the mold
cannot be identified by its conidia formation alone because
other fungal saprobes have similar conidia morphology.
At 37 degrees C the yeast form grows in about 7-10 d as a
buttery-like, soft colony with a tan color. Microscopically,
typical yeast forms are 12-15 microns in diameter with a thick cell
wall and a single bud with a characteristic wide base.
Laboratory conversion of forms of
growth
The yeast will convert
to the mycelial form when incubated at 25 degrees C, taking from 3 - 4
d or up to a few wks. Similarly, mycelial growth can be
converted to yeast form when incubated at 37 degrees C. Now it is
possible to take the mycelial growth (which is the easier to
grow), and confirm the isolate with a DNA probe in a matter of
h.
Histopathology
B. dermatitidis produces both a granulomatous and
suppurative tissue reaction.
Serology
Immunodiffusion test (precipitins in agar gel). The active
antigen is “A”, or “BAD-1”, an adhesin. Antibody concentrations
require 2 to 3 weeks to be high enough to cause a positive
precipitin reaction. This test is positive in about 80% of the
patients with blastomycosis. When positive, there is close to
100% specificity.
Complement fixation (CF) test
This test requires 2 to 3 mo after the onset of disease to
develop detectable antibodies. Besides this long delay another
disadvantage of the CF is that it cross-reacts with other fungal
infections (coccidioidomycosis and histoplasmosis). The
advantage is that it is a quantitative test so the patient's
response to therapy can be monitored over time.
Enzyme Immunoassay for antibodies
Microtitration plate EIA detects antibodies to BAD-1, a surface
antigen of B. dermatitidis (Richer et al, 2014). This assay is
more sensitive than the agar immunodiffusion test and is highly
specific for B. dermatitidis, with no cross-reaction in serum
from patients with histoplasmosis. The test is not, as yet,
commercially available.
Antigenuria
The urine of patients with blastomycosis may contain
cross-reactive or shared antigens with H. capsulatum. Patients
with multisystem disseminated disease have a high rate of
positive urine antigen detectable by EIA. So it has high
sensitivity but low specificity. Antigenuria was detected in
89.9% of patients with culture- or histopathology-proven
blastomycosis (Connolly et al, 2012). Specificity was 99.0% in
patients with non-fungal infections and healthy subjects, but
cross-reactions occurred in 95.6% of patients with
histoplasmosis.
Therapy (for
complete guideline see Chapman et al., 2008)
- Mild to moderate pulmonary or disseminated
blastomycosis: Itraconazole 200 mg oral tablets once or 2x/d for
6-12 mo.
- Moderately severe pulmonary or disseminated (but without CNS
involvement). Amphotericin B (AmB) or lipid AmB for 1-2 wks,
followed by itraconazole for 6-12 mo.
- CNS blastomycosis: lipid AmB for 4-6 wks, then oral azole
for at least 1 y (itraconazole, fluconazole or voriconazole).
- Immunosuppressed patients: Induction therapy with AmB or
lipid AmB followed by itraconazole for 12 mo. Therapy to
continue beyond 1 y if immunocompetence does not improve.
COCCIDIOIDOMYCOSIS
Introduction
Disease Definition
Coccidioidomycosis (a.k.a.:“Valley Fever”, “desert rheumatism”,
“cocci”) is primarily a pulmonary disease classed as a type of
community acquired pneumonia. It is caused by the inhalation of
airborne arthroconidia of the dimorphic fungus Coccidioides, found
in soil of the endemic areas in the climate of the lower Sonoran
life zone. That includes the central valley of California (including
Fresno, Kern, and King counties) and the Arizona endemic area
including Maricopa County (Phoenix) and Pima County (Tucson),
overlapping the border into NW Mexico. It is a New World disease.
Most people (60%) have no or mild flu-like symptoms and do not see a
doctor. Symptoms, when present, are fatigue, cough, fever, night
sweats, loss of appetite, chest pain, muscle and joint aches
particularly of ankles and knees. There may be a rash resembling
measles or hives but more often as tender red bumps on the shins or
forearms.
Range of Valley Fever Cases:
- Mild - 60%, not requiring medical attention.
- Moderate - 30%, requires medical attention
- Complications - 5% to 10% (see Clinical Forms)
- Fatal - less than 1%
After recovery most people will have life-long immunity.
Coccidioidomycosis is not communicable.
Diagnosis
Physical diagnosis consists of checking for fatigue, respiratory,
musculoskeletal symptoms, and skin rashes. History of residence in
or recent travel to the endemic areas is queried*. If the answers to
the above questions are positive then coccidioidomycosis is in the
differential and pertinent tests are requested. The first
presumptive test is an EIA screening test for IgM or IgG
coccidioidal antibodies. If positive, risk factors and complications
are queried. A negative test does not exclude coccidioidomycosis. If
a follow-up tests remain negative over 2 mo the probability of
coccidioidomycosis is lower. Additional confirmatory lab tests,
including culture of the pathogen, are discussed under “Laboratory”,
below.
*Uncommonly, handling goods originating in the endemic areas.
Etiologic Agents
Coccidioides immitis (California); C. posadasii (Arizona, Mexico,
microfoci in Latin America). Soil dwelling mold, rapid growing,
fluffy or powdery colony (buff, yellow or tan). Hyphae fragment into
arthroconidia: They are the infectious particles, dangerous to
inhale!
Life cycle of Coccidioides spp. (fig. 6)
Coccidioides is a dimorphic
fungus with two distinct forms. 1) Environmental form: the
infectious propagules are arthroconidia formed by fragmentation of
hyphae in soil. 2) The tissue form consists of endosporulating
spherules.
 Fig.6. Biology of coccidioidomycosis .
Fig.6. Biology of coccidioidomycosis .
Source: CDC
Geographic Distribution
The geographic distribution of Coccidioides spp. is the Sonoran
desert, which includes the deserts of the Southwest (California,
Arizona, New Mexico, Nevada, Utah and Texas) and northern Mexico
(fig. 6). It is also found in small foci in Central and South
America. The climate is the Lower Sonoran life zone consisting of
arid and semi-arid desert and desert grassland with hot summers and
a few winter freezes, low altitude, and alkaline soil.
Characteristic plants are creosote bush (Larrea tridentata), Joshua
tree (Yucca brevifolia), and cacti. Other typical plants include
Black Grama (Bouteloua eriopoda), Lechuguilla (Agave lechuguilla),
Tarbush (Flourensia cernua), and Ocotillo (Fouquieria splendens).
Some typical mammals include Merriam's Kangaroo Rat (Dipodomys
merriami) and Mearns Grasshopper Mouse (Onychomys arenicola). Rain
storms occur in “Monsoon” season, in July – September.
Ecologic Niche
Desert soil, pottery, archaeologic middens, cotton grown in the
endemic areas, and rodent burrows all can harbor Coccidioides spp.
mold forms. Spores, arthroconidia, of the fungus are readily
airborne, can be carried by the wind, spreading hundreds of miles in
storms. In 1978, cases were seen in Sacramento 500 miles north of
the endemic area from a dust storm in southern California.
Epidemiology
Seasonality in the Arizona endemic area (fig. 7) (Comrie 2005,
Komatsu et al., 2003). Seasonal patterns in the desert southwest
U.S. coincide with peaks and off-peak for coccidioidomycosis.
Arizona experiences about 12.5 inches of annual rainfall but it
follows a bimodal seasonality. Winter has wet weather
(December-March), followed by the driest time of year: foresummer
(May-July), monsoon season of wet weather (August-September), fall
is dry (October-December). Coccidioidomycosis seasons for exposure
consist of a winter decrease (January through April), a foresummer
peak (May –July), a monsoon decrease (August-September), and a fall
peak (October-December).
 Fig. 7. Seasonality of coccidioidomycosis in the AZ endemic area
(see text for details)
Fig. 7. Seasonality of coccidioidomycosis in the AZ endemic area
(see text for details)
Source: Komatsu et al, 2003 CDC
Monthly coccidioidomycosis rates are consistent with increased dust
exposure leading to increased disease incidence. Precipitation
during the foresummer is most favorable for Coccidioides growth at a
time when the soil is desiccated and vegetation is dormant. Fungal
spores produced after a wet period in the foresummer may remain
viable in the soil for years.
Seasonality in the California endemic area. The center is
Bakersfield. Wettest mo are Jan-March. May –Sept are dry, with
precipitation increasing from October to December. Annual cycles of
valley fever incidence and climate variables in San Joaquin Valley,
California are shown in fig 8 (Gorris et al., 2017).
Climate factors other than seasonality influence coccidioidomycosis:
An outbreak of coccidioidomycosis occurred after the 1994 earthquake
in Northridge, California with an attack rate of 30 cases per
100,000 inhabitants. Being in a dust cloud increased the risk of
diagnosis (Schneider et al., 1997). A dust storm originated near the
Tehachapi mountains in southern California (Pappagianis and
Einstein, 1978). This ground level windstorm of December 20, 1977
carried heavy quantities of dust containing Coccidioides
arthroconidia to the north and west of Kern County in the San
Joaquin valley, CA. In the following 5 mo new coccidioidomycosis
cases occurred in northern and coastal areas of San Francisco,
Sacramento, the east bay, and Santa Clara and Monterey counties. By
the end of the May 1978, more than 532 new cases of
coccidioidomycosis were confirmed by the California State Department
of Health.
 Fig. 8. Mean annual cycles of valley fever incidence and climate
variables in San Joaquin Valley, California. (a) Monthly valley
fever incidence, (b) surface air temperature, (c) monthly
precipitation, (d) avg. soil moisture in the top 10 cm, (e) surface
dust conc. Incidence reaches seasonal maxima following periods of
low environmental moisture. Error bars are s.d. of mo avg between
counties (Gorris et al., 2017).
Fig. 8. Mean annual cycles of valley fever incidence and climate
variables in San Joaquin Valley, California. (a) Monthly valley
fever incidence, (b) surface air temperature, (c) monthly
precipitation, (d) avg. soil moisture in the top 10 cm, (e) surface
dust conc. Incidence reaches seasonal maxima following periods of
low environmental moisture. Error bars are s.d. of mo avg between
counties (Gorris et al., 2017).
Used with permission of a Creative Commons license for educational
purpose
Incidence and Prevalence
California endemic area
From 1995, when coccidioidomycosis became an individually
reportable disease in California, to 2009, annual incidence
rates ranged from 1.9 to 8.4 per 100,000, followed by a
substantial increase to 11.9 per 100,000 in 2010 and a peak of
13.8 per 100,000 in 2011. Annual rates decreased during
2012–2014, but increased in 2016 to 13.7 per 100,000, with 5,372
reported cases, the highest annual number of cases in California
recorded to date (Cooksey et al., 2017).
Arizona endemic area
In the same period as the above report in the Arizona endemic area
incidence rates rose from 36.1 per 100, 000 in 1999 to 255.8 per
100,000 in 2011 and in 2016 the rate was 89.3 per 100,000 with
6101 reported cases (Arizona Department of Health Services,
2017)
Occupational and
recreational risk factors for coccidioidomycosis
(Freedman et al., 2018).
Human activity in the California and Arizona endemic areas are
associated with outbreaks of coccidioidomycosis. Among these are
military maneuvers, construction, archeologic digs, including
disruption of Native American sites.
Outbreaks have occurred among persons incarcerated in the endemic
areas who often had no prior exposure to coccidioidomycosis, were
immunologically naïve, and thus at greater risk. To minimize
illness, inmates who are immunosuppressed, are African-American, or
Filipino, or have diabetes mellitus are no longer housed in several
prisons in California’s Central Valley.
Cases remote from the endemic area are usually in patients who have
visited an endemic area and brought back pottery, or blankets
purchased from a dusty roadside stand, or in Navy and Air Force
personnel who were exposed when they were stationed in the endemic
area. An example is of cases occurring in cotton mills in Burlington
and Charlotte, N.C. when cotton, grown in the desert of the
southwest U.S, was contaminated with fungus spores and mill workers
inhaled them while handling the raw cotton.
Risk Groups/Factors
Life-long immunity usually follows infection with Coccidioides
spp.
Risk factors for
Coccidioidomycosis
Risk of disseminated coccidioidomycosis linked to ethnicity.
Single-site and multisite disease accounted for 86% of
extrapulmonary Coccidioides infections in African-Americans and 91%
in Asians but for only 56% in whites and 52% in Hispanics (Odio et
al., 2017). Further, African-Americans accounted for about one-third
of single-site and multi-site infections while making up only 6% of
the population in coccidioidomycosis-endemic areas. In contrast,
only 10% of patients with single-site and multi-site disease were
Hispanic, even though Hispanics are 35% of the population in those
areas.
Exogenous immunosuppression is a more significant factor than
intrinsic racial/ethnic variation in host defense. Future studies
with known ancestral markers will help identify associations between
coccidioidomycosis and race/ethnicity.
Occupational risk factors
Workers in endemic areas at increased risk for
coccidioidomycosis include those employed in agriculture,
construction, and archeologic work, military personnel, and
those in mining, quarrying, and oil and gas extraction
industries (de Perio et al 2019). The common theme is
disturbance of the soil, or dust-disturbing winds. Clusters of
infections also occurred among employees and inmates at state
prisons in endemic areas. Newer industries such as solar farms
also expose workers. Coccidioidomycosis is also known as a
troubling risk in clinical laboratories.
Transmission
Inhalation of Coccidioides spores (arthroconidia)
carried in dust particles from the soil by the wind when the
desert soil is disturbed in the two defined endemic areas:
Central Valley of California and in Arizona .
Determinants of Pathogenicity
Dimorphism. The organism follows the saprobic cycle in the soil
and the parasitic cycle in humans and animals. The saprobic
cycle starts in soil with arthroconidia that develop into
mycelium. The mycelium then matures and forms alternating
arthroconidia which, when released, germinate back into mycelia
(fig 9). The parasitic cycle involves the inhalation of the
arthroconidia by animals which then form spherules filled with
endospores (fig 10).
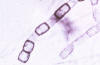 Fig. 9. Coccidioides alternating arthroconidia; these are the
infectious propagules, dangerous to inhale.
Fig. 9. Coccidioides alternating arthroconidia; these are the
infectious propagules, dangerous to inhale.
Source: Dr. Arthur F. DiSalvo
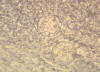 Fig. 10. Smear of exudate showing spherules of Coccidioides immitis.
Experimental infection of mouse with soil sample.
Fig. 10. Smear of exudate showing spherules of Coccidioides immitis.
Experimental infection of mouse with soil sample.
CDC
Mechanisms permitting the survival of
Coccidioides spp. in the
host tissues
(Hung et al., 2007).
Spherule outer wall
glycoprotein (SOWgp) compromises T- cell-mediated immunity. When
SOWgp is depleted on the surfaces of endospores, host
recognition of the pathogen is prevented. The endospores induce
elevated host arginase I and also induce coccidioidal urease,
both of which contribute to local tissue damage. Arginase I
competes with inducible nitric oxide synthase in macrophages for
the common substrate, L-arginine, thereby reducing nitric oxide
(NO) production and increasing the synthesis of host ornithine
and urea.
Host-derived L-ornithine may promote pathogen growth and
proliferation by providing a pool of the monoamine, which could
be used to synthesize polyamines via metabolic pathways of the
fungal cells.
High concentrations of Coccidioides- and host-derived urea at
infection sites in the presence of urease produced by the
pathogen, results in secretion of ammonia, alkalinizing the
microenvironment. Ammonia and active urease released from
spherules during the parasitic cycle of Coccidioides increase
the severity of coccidioidal infection by compromising the
immune response to infection and by damaging host tissue at foci
of infection.
Clinical Forms
- About 60 % of infections in the endemic area are asymptomatic.
- Another 25 % of exposed people suffer a "flu-like" illness and
recover without therapy. They have typical symptoms of a
pulmonary fungal disease: anorexia, weight loss, cough, hemoptysis. The average recovery time is wks to mo. in
immune-normal people.
- About 5% of Valley Fever pneumonias result in the development
of lung nodules: small residual patches of infection appearing
as solitary lesions, typically 1 - 1.5 in diam. often with no
symptoms. On a chest x-ray, these nodules can resemble cancer.
- An additional 5% of patients develop lung cavities after an
initial infection, occurring most often in older adults, usually
without symptoms, and about 50% of cavities disappear within two
years. Occasionally, cavities rupture, causing chest pain and
difficulty breathing, requiring surgery.
- Of patients who seek medical attention 1-2% percent develop
disseminated disease; the most common site of dissemination is
the skin. Skin biopsy may reveal Coccidioides when grown in
culture.
- Bones and joints (especially knees, vertebrae, and wrists) are
frequent sites of dissemination.
- Meningitis is the most serious and potentially lethal form of
disseminated disease, with headache, vomiting, stiff neck, and
other CNS disturbances. A lumbar puncture is used to diagnose
meningitis.
Therapy
Many patients with Valley Fever pneumonia do not require
antifungal therapy because the infection is controlled by the
normal immune response. Patients who develop acute and severe
pneumonia require specific therapy. Other patients may develop
progressive pulmonary or extrapulmonary dissemination. These
latter two classes of patients both require treatment (Galgiani
et al., 2016, Galgiani and Thompson III 2016).
Factors affecting increased susceptibility include: diabetes,
advanced age, other comorbidities.
A majority of patients with complicated coccidioidomycosis have
a subacute or chronic clinical course. Initial therapy with oral
fluconazole (or itraconazole) may be necessary to continue for
months or more. Relapses may occur in approximately one-third of
patients who are apparently cured. In that event lifetime
maintenance therapy may be required. That category of patients
may have T-cell mediated immune deficiency or disease that
progressed to meningitis.
Symptomatic cavitary coccidioidal
pneumonia
Primary therapy
with fluconazole or itraconazole. A surgical option is
considered if the cavity or cavities remain symptomatic despite
antifungal treatment. A ruptured coccidioidal cavity is a
further complication. Galgiani et al., 2016 discuss the
preferred approach to surgical management of these conditions.
Coccidioidomycosis of the bones,
joints
Azole therapy is
recommended but if there is extensive or limb-threatening
skeletal or vertebral disease AmB is recommended as primary
therapy switching to long-term azole therapy after a
satisfactory clinical response. Vertebral coccidioidomycosis
warrants a surgical consultation.
Coccidioidal meningitis
Fluconazole is recommended as initial
therapy. Itraconazole may be used but requires close monitoring
to ensure adequate absorption. If patients fail initial therapy
with fluconazole, another oral azole or intrathecal amphotericin
B should be considered. Lifetime azole treatment is recommended
for this clinical form.
In rapidly progressive coccidioidomycosis, AmB is preferred as
initial treatment, stepping down to azole upon satisfactory
clinical response. Azole therapy may be needed for the lifetime
of the patient to maintain control.
Laboratory
Immunodiffusion tests
IgM react with a polysaccharide antigen
in the fungal cell wall. The IgG test reacts with a specific
chitinase enzyme. Immunodiffusion tests are considered
confirmatory of EIA test results.
Serology for coccidioidomycosis is conducted by the
coccidioidomycosis serology laboratory at the University of
California Davis, George Thompson M.D., director.
- Qualitative: Immunodiffusion (ID) to determine coccidioidal
IgM ("precipitin") (test termed “IDTP”), or IgG (CF) (test
termed “IDCF”) antibody in body fluids. This is appropriate if
no diagnosis of coccidioidomycosis has previously been
established or to confirm an EIA test result.
- Quantitative: Determination of IgG titer by complement
fixation (CF) is appropriate after a diagnosis has been made by
positive ID test or culture of the organism from patient
specimens. A positive CF test in a single specimen can be
significant. Importantly, sequential CF tests can monitor
response to therapy.
- Interpretation.
- ID tests. A positive IDTP test for IgM or IDCF for IgG. The
IgM is important in early diagnosis of acute primary
coccidioidomycosis, and is positive in most patients 1 to 2 wks
after onset of symptoms, may persist several mo. or longer when
there are pulmonary cavities or extrapulmonary dissemination.
Detecting IgM in the CSF is associated with coccidioidal
meningitis. The IDCF for IgG can be associated with recent
infection but also detectable in serum mo or yrs later.
- Complement fixation (CF). A rising serum CF titer greater than
1:16 is often associated with dissemination, but lower titers
can occur with dissemination limited to single lesions or
meningitis. A titer of greater than 1:128 usually indicates
extensive dissemination. The CF test is prognostic: A rising
titer indicating a poor response, whereas a dropping titer
reflects a favorable response.
- CF positive CSF specimens usually indicate meningitis, but IgG
can be detected in the CSF in the absence of meningitis. The CF
test of CSF may initially be negative in coccidioidal meningitis
in about 5% of patients.
- Negative serologic tests do not exclude coccidioidal disease
in some persons living with AIDS or in other immunocompromised
patients.
Enzyme linked immunoassay (EIA)
The Omega EIA (IMMY
laboratories, Norman, OK) is a qualitative serum test using two
different well strips. One is coated with a heat stable antigen
and measures the IgM response, corresponding to the classical TP
test, indicating early acute coccidioidomycosis. The second well
strip contains heat labile chitinase antigen and measures the
IgG response, the same antibodies measured in the classical CF
test. A single 1/441 dilution of patient serum is tested. The
EIA tests are read to an endpoint.
MVista® Coccidioides Quantitative Ag EIA
(MiraVista
Laboratories, Indianapolis IN) (Durkin et al., 2009). This is a
sandwich EIA that detects a heat stable antigen, a presumptive
galactomannan.
Pretreatment of serum samples with EDTA at 100°C improved the
sensitivity of detecting Coccidioides antigenemia. Antigenemia
was detected in 28.6% of patients whose samples were not EDTA-heat
treated and in 73.1% of those whose samples were treated.
Antigenuria was detected in 50% of patients. Specificity of 100%
was obtained in healthy subjects, but cross-reactions were seen
in 22.2% of patients with histoplasmosis or blastomycosis.
The MVista Coccidioides EIA was evaluated in CSF 36 patients
with coccidioidal meningitis (Kassis et al., 2015). Sensitivity
and specificity as reported were 93% and 100%, respectively.
Specificity was tested against 88 patients in the Maricopa
County, AZ health system, who had CSF abnormalities consistent
with meningitis owing to other causes. In the group with confirmed
coccidioidal meningitis, cultures of CSF were positive in 7%,
antibodies were demonstrated by complement fixation and
immunodiffusion of between 67%-70%, which increased to 85% using
an EIA to detect IgG.
.
Real-time PCR detection of Coccidioides spp.
DxNA LLC, St.
George, Utah
received FDA approval to market GeneSTAT. MDx
Coccidioides test in December 2017. This PCR based test is available for use on site in the clinical
laboratory, with a same day result. The assay is performed on
broncho-alveolar lavage (BAL) or bronchial wash (BW) specimens.
The BAL/BW sample preparation includes the lysis of Coccidioides
spherules in the sample with sputolysin, then DNA extraction and
purification with the QIAGEN QIAamp DSP DNA Mini Kit. PCR of the
target sequence is done in real time. DxNA completed a
multi-center clinical study at 3 centers in AZ, and New Mexico,
also including California samples to compare the GeneSTAT Valley
Fever assay to culture of C. immitis and C. posadasii.
Direct examination and Culture of Coccidioides spp.
Clinical specimens include material from: biopsy of skin
lesions, blood, bone, brain, bronchial washings, bronchoalveolar
lavage, CSF, joints, pus from skin lesions, sputum. Viscous
mucoid sputum can be liquefied by treatment with dithiothreitol
in phosphate buffer at pH 7.0 (Hardy Diagnostics) and then
centrifuged to recover Coccidioides for direct exam and culture.
Direct Examination
For microscopy, specimens are mounted in 10% KOH with or without Calcofluor white fluorescent dye. The
Coccidioides tissue form consists of thick-walled spherules,
varying in size 30- 60 μm diam., filled with endospores (2–5 μm
diam.). Spherules with endospores are diagnostic. (see
histopathology in fig.11). Mature spherules rupture releasing
endospores which, during infection, develop into more spherules.
Conversion of the mold into the spherule form is not possible in
the clinical lab.
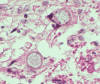 Fig. 11. Histopathology showing Coccidioides spherules in lung
tissue with endospores H&E stain.
Fig. 11. Histopathology showing Coccidioides spherules in lung
tissue with endospores H&E stain.
Photo credit: used with
permission from Dr. Kenneth G. Van Horn
Culture
As mentioned,
C. immitis and C. posadasii are
dimorphic. (fig 6). Colonies on Sabouraud Dextrose agar
incubated at 25 o - 30o C grow in the mold form within 3-5 d,
and usually sporulate in 5-10 d. Another medium choice is Brain
Heart infusion agar. Addition of cycloheximide to the medium for
growth inhibits saprobic fungi. The highly infectious fungus can
cause laboratory infections, agar slant tubes with screw caps
are much preferred to Petri plates for primary isolation. In the
event that Petri plates are used, they should be sealed with gas
permeable tape (Shrink Seals® Scientific Device Laboratory (YouTube
video), Shrink-Seal (Remel),
or MycoSeal®, Hardy Diagnostics), and examined only in a
biological safety cabinet. Unlike bacteriologic practice,
mycologists should never sniff any fungal cultures. Because of
the infectious nature of Coccidioides species, serology or
direct exam, instead of culture, are preferred diagnostic
methods.
In culture, mycelia fragment into arthroconidia: barrel-shaped
(smaller at the edges, wider at the middle) asexual spores.
Arthroconidia alternate with non-spore-forming (“disjunctor”)
cells in the mycelium, giving rise to the term “intercalary”
arthroconidia. Fragments of the adjacent cells remain attached
to the arthroconidia giving the appearance of “wings”.
Histopathology
The inflammatory reaction is both purulent and granulomatous.
Recently released endospores evoke a polymorphonuclear response.
As endospores mature into spherules, the acute reaction is
replaced by lymphocytes, plasma cells, epithelioid cells and
giant cells. Spherules of various sizes (10 -100 µm) with many
endospores (2 to 5 µm) are hallmarks of coccidioidomycosis and
can be observed with H&E stain (fig 11). GMS stains both
spherule walls and endospores. Endospores are released into the
tissues when spherules rupture. Sometimes mycelia of
Coccidioides are found in cavitary lung or skin lesions (Guarner
and Brandt, 2011)
The inflammatory response to endospores is mainly neutrophilic,
whereas reaction to spherules is granulomatous. Early after
infection lesions are pyogranulomas because of the high
concentration of spherules and endospores . Lymphocytes cluster
around granulomas with necrosis are an important response to
coccidioidomycosis .
Eosinophils may be present leaving an eosinophilic matrix around
spherules, the Splendore-Hoeppli reaction.
Differential histopathology
Rhinosporidium seeberi, produces
large sporangia (larger than Coccidioides spherules} with
internal endospores. Endospores released from spherules or young
spherules without endospores can be mistaken for Blastomyces,
Histoplasma, Emmonsia, Candida, Pneumocystis, other yeasts. In
immunosuppressed patients Pneumocystis and Coccidioides may
occur in the same specimen.
HISTOPLASMOSIS (Histoplasma capsulatum)
Introduction
Disease Definition
Histoplasmosis is a community-acquired
pulmonary infection that, before it is contained, can spread to
organs of the mononuclear phagocytic system: bone marrow, liver,
and spleen. Fig. 12 shows yeast forms phagocytosed by a
macrophage. Hepatosplenomegaly is the primary sign in children.
The agent is Histoplasma capsulatum, occurring in soil mixed
with bird or bat feces, e.g.: blackbird roosts, chicken coops,
caves. Exposure is common in residents of the endemic area who
encounter it from disturbing the soil. The endemic area includes
states bordering the Mississippi and Ohio River valleys,
extending to eastern Canada, but is expanding as a result of
climate change, as discussed below. Most exposure is subclinical
but depending on the inhaled dose and the immune system of the
exposed person may result in pneumonia and, in a smaller group,
to extrapulmonary dissemination.
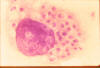 Fig. 12. Giemsa- stained tissue smear from a human case shows a
macrophage with phagocytosed H. capsulatum yeast forms.
Fig. 12. Giemsa- stained tissue smear from a human case shows a
macrophage with phagocytosed H. capsulatum yeast forms.
Source CDC,
Dr. D.T. Mc Clenan, ST69-2228
Diagnosis
The diagnosis is made from the patient’s history,
diagnostic imaging, isolation and culture of the organism, and
via serology and a commercial DNA test.
Differential Diagnosis: Tuberculosis, other bacterial
pneumonias, lung tumor (in the case of a solitary pulmonary
nodule), sarcoidosis.
Etiologic Agents
Histoplasma capsulatum is classed in the family
Ajellomycetaceae, order Onygenales, Ascomycota. Histoplasma
capsulatum is a haploid organism and has a heterothallic mating
system. In clinical samples the (-) mating type predominates.
The non-repetitive “core” Histoplasma genome is roughly 26–28 mB
encoding 9,000-10,000 genes.
H. capsulatum is divided into geographically distinct lineages,
including 6 major clades: North American class 1 (Nam1), North
American class 2 (Nam2), a Panamanian clade, Latin American
group A (LamA), Latin American group B (LamB), and an African
clade (including variety duboisii) (Edwards and Rappeleye,
2011).
Geographic Distribution - Ecologic Niche
Geographic Distribution According to an oft cited 1969 map
(Edwards et al., 1969) showing histoplasmin skin test reactivity
among Navy recruits in the U.S., the prevalence of H. capsulatum
matched states bordering the Mississippi and Ohio River valleys.
Since then change has occurred in the envi¬ronment, climate, and
human population trends (Maiga et al., 2018) (fig.13). The
advent of AIDS and of immunosuppressive therapy revealed unknown
endemic areas. Outbreaks in Montana and Nebraska led CDC to
propose that histoplasmosis is now endemic there. State
incidence rates of histoplasmosis among older persons also show
a shift in the direction of Nebraska and northern Great Lakes
regions. Surveillance from 2011- 2014 in 12 states points to
other previously unknown endemic areas in Minnesota, Wisconsin,
and Michigan.
Ecologic niche
Blackbird roosts, chicken houses and bat guano.
A patient may have spread chicken manure around his or her
garden and 3 wks later developed a pulmonary infection.
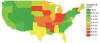 Fig. 13. State-level histoplasmosis incidence rates for 1999–2008 US
Medicare and Medicaid data (no. cases/100,000 person-years), IR,
incidence rate. Source: Maiga AW, Deppen S, Scaffidi BK, Baddley J,
Aldrich MC, Dittus RS, Grogan EL. Mapping Histoplasma capsulatum
exposure, United States 2018 Emerg Infect Dis. 24:1835-1839
Fig. 13. State-level histoplasmosis incidence rates for 1999–2008 US
Medicare and Medicaid data (no. cases/100,000 person-years), IR,
incidence rate. Source: Maiga AW, Deppen S, Scaffidi BK, Baddley J,
Aldrich MC, Dittus RS, Grogan EL. Mapping Histoplasma capsulatum
exposure, United States 2018 Emerg Infect Dis. 24:1835-1839
Image credit: Stephen Deppen, Department of Thoracic
Surgery, 609 Oxford House, 1313 21st Ave S, Nashville, TN 37232,
USA; email: steve.deppen@vanderbilt.edu
Epidemiology, Incidence and Prevalence
A summary of histoplasmosis outbreaks in the U.S.A. over the
period 1938-2013 is in Benedict and Mody 2016.
Cases of histoplasmosis are only reportable to public health
authorities in 10 states. Because of that the true incidence is
uncertain. Surveillance data for 2011–2014 from 13 states were
studied by Armstrong et al., 2018: Alabama, Arkansas, Delaware,
Illinois, Indiana, Kentucky, Michigan, Minnesota, Mississippi,
Nebraska, Ohio, Pennsylvania, Wisconsin. These data revealed a
total of 3,409 cases. Of these reports 1,273 (57%) patients were
hospitalized, and 76 (7%) patients died. Three states reported
cases associated with an outbreak (816 patients). Median
hospitalization stay was an estimated 7d (range 1–126 d).
Exposure to bird or bat droppings in the weeks before symptoms
developed was reported by some states. e.g.: In Michigan bird
and/or bat droppings were cited in 29% of their cases. Annual
incidence rates were highest for Arkansas, Illinois, Indiana,
Michigan, and Minnesota, ranging from 1.25 - 4 cases/100,000
population.
The extent of subclinical exposure is unknown because production
of the skin test reagent, histoplasmin, was discontinued in
2000, depriving the public health community of an important
epidemiologic tool.
Risk Groups/Factors
Anyone working a job or present at
activities where soil contaminated with H. capsulatum is
disturbed can develop histoplasmosis, depending on the number of
conidia inhaled and a person’s age and susceptibility. The
number of inhaled conidia to cause disease is unknown. Children
younger than 2 y-old, persons with weakened immune systems,
older persons, especially those with diabetes or chronic lung
disease, are at increased risk to develop symptomatic
histoplasmosis. The high-risk group also includes persons living
with AIDS, or cancer, and those receiving chemotherapy, high
dose, long-term steroid therapy or other immunosuppressive
drugs.
The proportion of hospitalizations for immune-mediated
inflammatory disease (rheumatoid arthritis, inflammatory bowel
disease, and psoriasis) listed on discharge records also
increased from 4% in 2001 to 10% in 2012, as did the proportion
with solid organ or stem cell transplant (from 1% to 6%)
(Armstrong et al., 2018).
A previous infection can provide partial immunity to reinfection.
Recreational or
occupational risk
In bat caves of Mexico and
Central America histoplasmosis is a recreational disease of cave
explorers and an occupational disease of workers who harvest bat
guano for fertilizer. Here is a summary of occupations and
recreation activity that carry an increased risk of exposure to
H. capsulatum:
- Bridge inspector or painter
- Chimney cleaner
- Construction, demolition, and maintenance workers
- Farmer
- Gardener
- Heating and air-conditioning system worker
- Microbiology lab worker
- Pest control worker
- Restorer of historic or abandoned buildings
- Roofer
- Spelunker (cave explorer)
Outbreak
An outbreak occurred in South Carolina, U.S.A. when
workers used bulldozers to clear canebrakes which served as
blackbird roosts (DiSalvo and Johnson 1979). All who were
exposed, workers and bystanders, contracted histoplasmosis. Soil
studies found viable H. capsulatum persisted at declining levels
over a 9-y period. Skin tests showed that 27.3% of 8th grade
students who resided in a 20 km radius of the contaminated site,
who were lifelong residents of the same dwelling, had a positive
histoplasmin skin test.
Transmission
Inhalation of conidia after disturbing the soil
admixed with bird or bat feces. Soils may remain a source of
infection months or years after bird roosts are gone.
Determinants of Pathogenicity
(Edwards and Rappeleye 2011)
The mold form is avirulent and preventing the mycelia to yeast
switch at 37°C blocks pathogenicity.
Several genes regulate the transition to yeast form: a kinase
Drk1; the Wor1 homolog, Ryp1; and two velvet family regulators,
Ryp2 and Ryp3. As yet, only a few genes have been proven to
contribute to virulence in vitro or in vivo.
- Cbp1. A secreted protein, the loss of which in attenuates
virulence of yeast forms in macrophage and mouse assays. Its
role is to transform phagocytes into a permissive environment
for yeast replication.
- (1,3)-α-D-glucan. Chemotype II strain walls contains α- glucan
whereas chemotype I strains lack this polysaccharide. α-glucan
production is critical to virulence of chemotype II yeast. α-glucan
promotes virulence by preventing recognition by host immune
cells. The α-glucan polysaccharide forms the outermost yeast
wall layer and conceals cell wall ß-glucans that would normally
be detected by dectin-1 receptors on macrophages.
- Yps3 is a secreted and cell wall protein with sequence
homology to an adhesin of Blastomyces dermatitidis. The Yps3
protein attaches to chitin on the G217B yeast cell wall. Yps3
(-) yeast forms are deficient in dissemination to spleen and
liver.
- Iron acquisition. H. capsulatum has more than one pathway to
acquire iron. Siderophore deficient H. capsulatum have decreased
ability to assimilate iron and display stunted growth in
macrophages and decreased virulence.
- Adhesins. Cell-surface Hsp60 is an adhesin mediating
attachment of yeast forms to complement receptors on
macrophages.
- Catalase. Hydrogen peroxide metabolizing enzymes can block or
inhibit anti-microbial reactive oxygen. The Histoplasma
M-antigen corresponds to the CatB catalase protein.
- ß-Glucosidase. The Histoplasma H-antigen is produced by all
strains, but some yeast strains release over ten times as much
ß-glucosidase activity.
Clinical Forms
Asymptomatic exposure. In the endemic area the great majority of
patients who develop histoplasmosis (95%) are asymptomatic.
Histoplasmosis generally occurs in one of three forms: acute
pulmonary, chronic pulmonary, or disseminated.
- Acute Pulmonary. There is generally complete recovery from the
acute pulmonary form (a "flu-like" illness).
- Chronic Pulmonary. Symptoms include apical cavities and
fibrosis. Persistence of the organism leads to progressive
destruction and fibrosis. This form while, uncommon or rare, is
associated with people who have underlying pulmonary disease.
- Disseminated. Patients will first notice shortness of breath
and a cough which becomes productive. The sputum may be purulent
or bloody. Patients will become anorexic, lose weight and have
night sweats. If untreated this form of disease is usually
fatal.
- Radiographic and CT findings.
Pulmonary histoplasmosis may evoke lung granulomas, causing
false-positive readings of lung tumors in radio¬graphic images
on HR- CT and positron emission tomography (FDG-PET). This means
that an increased awareness of the conditions for H. capsulatum
infection is of epidemiologic and clinical importance.
The differential diagnosis includes tuberculosis, and the chest
x- ray also looks like tuberculosis, but radiologists can
distinguish them on the chest film (histoplasmosis usually
appears as bilateral interstitial infiltrates.)
Pulmonary histoplasmosis presents a wide array radiologic
findings, which can mimic other chest diseases, e.g.: primary
lung neoplasm, bacterial pneumonia.
- Acute pulmonary: solitary or multiple nodules, lymphadenopathy,
pleural effusion (fig. 14, Solitary pulmonary nodule), (fig 15,
multiple nodules)
- Disseminated: diffuse micronodular or air-space opacities.
- Chronic cavitary: chronic upper lobe consolidation with
progressive cavitation and volume loss (seen in emphysema
patients).
- Chronic: calcified pulmonary nodules, histoplasmoma, fibrosing
mediastinitis.
Please see Semionov et al., 2019 for illustrations of the above
conditions.
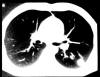 Fig. 14. A 40-year-old man with a persistent nodular density in the
left lower lobe. CT scan of chest 6 mo before admission.
Fig. 14. A 40-year-old man with a persistent nodular density in the
left lower lobe. CT scan of chest 6 mo before admission.
Source: Urschel Jr, HC, Mark EJ 1988 Case records of the Massachusetts
General Hospital. Weekly Clinicopathological Exercises. Case
49-1988: N Engl J Med. 319:1530-1537
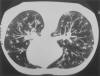 Fig. 15. Superior view of a transaxial CT scan of a patient’s
thoracic cavity, showing the classic “snowstorm” appearance of
pulmonary nodules in both lung fields, caused by the H.
capsulatum.
Fig. 15. Superior view of a transaxial CT scan of a patient’s
thoracic cavity, showing the classic “snowstorm” appearance of
pulmonary nodules in both lung fields, caused by the H.
capsulatum.
Source: CDC Public Health Image Library, #472
Therapy (for other clinical forms and dosage regimens please see
Wheat et al., 2007)
- Moderately severe to severe acute pulmonary histoplasmosis.
Lipid formulation of amphotericin B i.v. for 1–2 wks followed by
itraconazole for 12 wks.
- Mild-to-moderate acute pulmonary histoplasmosis. Antifungal
therapy is not usually necessary. Itraconazole 6–12 wks is
recommended for patients whose symptoms persist for >1 mo.
- Chronic cavitary pulmonary histoplasmosis. Itraconazole for at
least 1 y is recommended, but some prefer 18–24 mo because of
the risk of relapse.
- Pulmonary nodules (histoplasmomas). Antifungal treatment is
not recommended.
- Progressive disseminated histoplasmosis. For moderately severe
to severe disease, liposomal amphotericin B is recommended for
1–2 wks, followed by oral itraconazole for a total of at least
12 mo. For mild-to-moderate disease, itraconazole for at least
12 mo. Lifelong suppressive therapy with itraconazole may be
required in immunosuppressed patients.
Laboratory
Clinical specimens sent to the lab: Sputum, bronchial washings,
or bronchoalveolar lavage, or biopsy material from liver/spleen,
brain. Bone marrow is a very good source of the fungus, which
tends to grow in the mononuclear-phagocytic system. Peripheral
blood is also a source of histologic observation of the yeast
form is usually phagocytosed in monocytes or in PMN's (fig. 12).
An astute medical technologist performing a white blood cell
count may be the first to make a diagnosis of histoplasmosis. In
peripheral blood, H. capsulatum appears as a small yeast about
2-4 µm diam. (contrast to Blastomyces yeast forms: 12 – 15 µm
diam) Gastric washings are another source of H. capsulatum.
Fig.16 shows H. capsulatum yeast forms from an open lung biopsy.
 Fig. 16. Microscopic morphology of Histoplasma capsulatum yeast
form. Open lung biopsy stained with fluorescent antibody. Ellipsoid
cells with monopolar budding are 2-4 microns/diam.
Fig. 16. Microscopic morphology of Histoplasma capsulatum yeast
form. Open lung biopsy stained with fluorescent antibody. Ellipsoid
cells with monopolar budding are 2-4 microns/diam.
Source: E. Reiss
Mycology
When it grown on Sabouraud dextrose or Mycosel agars at 25 -30
degrees C, it appears as a white, cottony mycelium after 2 to 3
wks. As the colony ages, it becomes tan. In the mold form,
Histoplasma has a very distinct conidium: the tuberculate
macroconidium (fig.17). Microconidia are also produced. The
tubercles are diagnostic, but there are some non-pathogens which
appear similar. A medical mycologist will be able to distinguish
them. Cultures on BHI + 5% sheep blood and incubated at 37 deg.
C can grow the yeast form colony. The yeast cell is 2-4 µm diam
and ellipsoid in shape. This is not diagnostic. To confirm the
diagnosis of either yeast or mold forms use the DNA probe (Accuprobe®,
Hologics. Inc.)
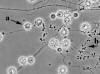 Fig. 17. Microscopic morphology of Histoplasma capsulatum mold form.
Tuberculate macroconidia (a) and microconidia, (b).
Fig. 17. Microscopic morphology of Histoplasma capsulatum mold form.
Tuberculate macroconidia (a) and microconidia, (b).
Source: E.
Reiss.
Serology
Serology for histoplasmosis is a little more
complicated than for other mycoses but provides more information
than blastomycosis serology. There are 4 tests: complement
fixation, immunodiffusion, EIA (antibody), EIA (antigen). The
serologic tests have different characteristics.
Complement fixation (C-F) test
The C-F test uses 2 antigens,
one from the yeast form and the other from the mycelial form.
Some patients react to only one; some react to both. C-F
antibodies develop later in the disease, about 2 to 3 mo after
onset. The C-F test cross reacts with other mycoses; it is
quantitative, and prognostic so the course of disease can be
followed with the C-F titer over time. Acute infection is
indicated by a 4-fold rise in antibody titers sera. A titer of
1:8 is positive, indicating previous exposure to H. capsulatum.
A titer of at least 1:32 or a 4-fold rise in antibody titer
indicates active infection.
Agar gel Immunodiffusion
Two immunoprecipitates may appear: the
H band is not commonly found but indicates active disease and
appears 2 to 3 wks after onset. An M band is frequent and
persistent, indicating past or present disease.
Prior to 2000 when the skin test was in use, the M-band might be
a result of a positive skin test. Skin tests also affect the C-F
titer. This interference is why skin tests are not used for
diagnosis.
Nucleic acid probe
Confirm mycelial isolates with DNA probe
recognizing ribosomal RNA, Accuprobe® Hologic, Inc., San Diego,
CA
Enzyme immunoassay
Antigen detection. Double Antibody Sandwich EIA (MiraVista
Laboratories, Indianapolis, IN). Specimens suitable for the
assay are urine, CSF, BAL, serum, plasma. Cross-reactions are
seen with blastomycosis, paracoccidioidomycosis, penicilliosis,
less in coccidioidomycosis, rarely in aspergillosis and
sporotrichosis.
Antibody detection
Indirect EIA in serum or CSF (MiraVista
Labs.) IgM and IgG versus Histoplasma antigens usually appear
during 1 mo of infection. IgM in acute pulmonary histoplasmosis
is detected in the acute phase (~3 wks) and declines in
convalescence, whereas IgG remains relatively constant at 6 wks.
Limitations are a12% cross reactivity with blastomycosis and 24%
cross reactivity with coccidioidomycosis.
Histopathology
Yeast forms are ellipsoid, 2- 4 µm diam., with a
narrow based bud. They are found phagocytosed by macrophages but
also seen in extracellularly. Gomori methenamine silver (GMS)
and periodic acid- Schiff (PAS) are the relevant stains for
formalin-fixed, paraffin-embedded tissue sections (fig. 18).
 Fig. 18. Histopathology of histoplasmosis. Gomori-methenamine-silver
(GMS) stain. Yeast forms stain black with a fast green background
that does not reveal the tissue reaction. Unevenly stained, single
and budding yeast forms x 350.
Fig. 18. Histopathology of histoplasmosis. Gomori-methenamine-silver
(GMS) stain. Yeast forms stain black with a fast green background
that does not reveal the tissue reaction. Unevenly stained, single
and budding yeast forms x 350.
Source: Fig. 194 Chandler FW, Watts JC, 1987 Pathologic Diagnosis of Fungal Infections. ASCP Press.
PARACOCCIDIOIDOMYCOSIS (Paracoccidioides brasiliensis, P. lutzii)
Introduction
Disease Definition
Paracoccidioidomycosis is a chronic
granulomatous disease of the lungs and mucous membranes of the
mouth endemic to Latin America. Subclinical exposure is common
in the endemic areas. Clinical forms include juvenile (subacute),
and chronic. Dissemination to the skin and visceral organs is
frequent. Regional lymph nodes are commonly involved. The
causative agents are the thermally dimorphic molds, Paracoccidioides brasiliensis, and
P. lutzii.
A common triad of symptoms consists of pulmonary lesions, gum
involvement with loosening teeth, (fig. 19) and cervical
lymphadenopathy (fig. 20).
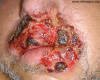 Fig. 19. Swelling of mouth and facial lesions,
paracoccidioidomycosis.
Fig. 19. Swelling of mouth and facial lesions,
paracoccidioidomycosis.
Source: Partners’ Infectious Disease Images; Accessed
on [06/14/2014] from http://www.idimages.org/ imageid=943. Case
#06010: A man from northern Brazil with respiratory symptoms and a
facial lesion. Available from: http://www.idimages.org/idreview/case/caseid=64.
Copyright Partners Healthcare System, Inc. All rights reserved
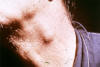 Fig. 20. Paracoccidioidomycosis, cervical lymphadenopathy.
Fig. 20. Paracoccidioidomycosis, cervical lymphadenopathy.
Source:
Dr. Arthur F. DiSalvo
Diagnosis
Paracoccidioidomycosis is diagnosed by demonstrating
P. brasiliensis in clinical samples including direct examination
and culture (please see Laboratory).Serology is a useful adjunct
and may be used to monitor the response to therapy.
- Confirmed cases: typical signs and symptoms (please see
Clinical Forms) and detection of P. brasiliensis yeast forms in
clinical samples.
- Probable: signs, symptoms and detection of antibodies in serum
by double immunodiffusion.
- Possible: Patient with at least 4 wks of cough, with or
without sputum and dyspnea, painful swallowing, hoarseness, skin
lesions, cervical or generalized lymphadenopathy. In children or
young adults with hepatosplenomegaly and/or abdominal mass.
- Differential. Paracoccidioidomycosis can often be misdiagnosed
as tuberculosis, although the two diseases can coexist.
Etiologic Agents
Paracoccidioides brasiliensis is a thermally dimorphic mold, and
a New World mycosis. It is an ascomycete classed in the
Onygenales and is genetically related to the other dimorphic
endemic mycotic agents: Histoplasma, Blastomyces, Coccidioides
species.
P. brasiliensis was considered the sole etiologic agent until
patient isolates from the central and western regions of Brazil
displayed different serologic and DNA fragment patterns leading
to the description of a separate species, P. lutzii.
Geographic Distribution/Ecologic Niche
The geographic
distribution of paracoccidioidomycosis is from mid-Mexico 20ºN
to Argentina 35ºS. Largest number of patients are from: Brazil,
Venezuela, Colombia, Ecuador and Argentina. About 80% of
recorded cases have occurred in Brazil. The lack of outbreaks,
long latency period, and movement of persons outside known
endemic areas contribute to uncertainty about the ecologic niche
of infection. P. brasiliensis has a known animal host in the
endemic areas: armadillos, Dasypus novemcinctus, P. brasiliensis
is recovered from armadillo tissues. Infected armadillos imply
the presence of the fungus in the local environment, even though
isolation from the environment is rare.
Epidemiology
Incidence and Prevalence he mean number of annual
cases was estimated for the period of 1930-2012 (reviewed by
Martinez, 2017). Most cases occurred in Brazil, varying from
21.6 cases/y in the NE to 207 cases/y in the SE of the country.
By comparison, in Argentina a total of 110 cases/y were recorded
during the same multi-year period. Hospitalization rates in
Brazil were 4.3/100,000 inhabitants/year during an 8 y period
ending in 2006. Mortality rates in Brazil were estimated at 1/ 1
million population/y giving a rough approximation of 209
deaths/y.
Typically, a case of paracoccidioidomycosis in the U.S. occurs
in someone who has worked in South America for some period of
time and then returned to the U.S. The patient may not realize
the importance of the history of travel to or residence in the
endemic area. Diagnoses of fungal diseases relies on careful
questioning and a probing history.
Risk Groups/Factors
Rural workers exposed to contact with the
soil are at greatest risk. This includes farmers and, in
particular, those working with coffee or tobacco crops. Exposure
of men and women to Paracoccidioides are nearly equal, as
revealed by skin tests, but men are disproportionately affected
because ß-estradiol inhibits growth of the yeast form of P. brasiliensis. Smoking is associated with the chronic form of
this disease. Immunosuppression including HIV infection increase
the risk of this infection.
Transmission
Aerosols containing mycelial fragments or conidia
are believed to be the mode of infection. No outbreaks of
paracoccidioidomycosis are reported, and the disease is not
communicable.
Determinants of Pathogenicity
The lungs are the usual portal of
entry for Paracoccidioides sp. (Mendes et al. 2017). Conidia
reach the alveoli, germinate, and convert to the yeast form,
causing pneumonitis. Then the fungus spreads to the regional
lymph nodes evoking a granulomatous response. The immune
response then determines disease progression. A satisfactory
response blocks infection at this stage, inflammation subsides
and the fungus is eradicated or remains in a latent stage which
may last for the life of the person or, after a long time, may
reactivate, triggering disease (see chronic form, in Clinical
Forms). If the immune response is insufficient in the initial
encounter the fungi multiply and spread via the lymphatic system
and hematogenously developing into the subacute form (see
Clinical Forms).
Gp43, a 43 kDa surface glycoprotein that functions as an adhesin
has been adapted for use as a skin-test antigen, can also be
detected in serum during infection. Some strains of P. brasiliensis do not express the antigen. Gp43 is not entirely
specific because the carbohydrate chains in gp43 cross react
with serum from patients with histoplasmosis and lacaziosis.
Gp43 enhances pathogenicity by inhibiting phagocytosis and
intracellular killing (Camacho and Niño-Vega 2017). Gp43
strongly induces in vitro granuloma-like formation by
macrophages. In the case of P. lutzii, a gp43 ortholog, “Plp43”,
shares only few epitopes in common; thus gp43 should not be used
in the diagnosis of patients infected with P. lutzii
α- 13- D-glucan is expressed in the yeast form. It enhances
pathogenicity by masking recognition of the major cell wall
polysaccharide: ß-13- D-glucan by the dectin-1 receptor of
macrophages. Dectin-1 is a pattern recognition receptor that
recognizes ß-glucan, triggering phagocytosis and downstream
release of cytokines.
The enlarged multi-budding yeast form of
Paracoccidioides
physically impairs phagocytosis.
Clinical Forms (Shankar et al., 2011)
Subclinical Positive skin test reactivity to paracoccidioidin or
serologic tests (anti gp43 antibodies) detected previous
subclinical infections in a significant proportion of healthy
individuals in various endemic countries (Martinez 2017). This
primary exposure is usually self-limited. Paracoccidioidomycosis
disease is thus regarded as affecting a small minority of
exposed infected individuals.
Juvenile
Children, adolescents, young adults (under 30 y-old).
In a sub-acute form there is lymph node enlargement, hepatomegaly, splenomegaly, and it may disseminate including
skin lesions, osteoarticular involvement. Rarely progresses to
chronic form. May reactivate years later.
Chronic
Seen in adults over 30 y-old, may result from
reactivation of quiescent lesions. The chronic pulmonary form
may be mild to severe. Disease progression is slow. Without
treatment, the severe form may ensue: Painful lesions on the
mucous membranes of mouth, lips, gums, and on the skin. There is
wt loss, lymphadenopathy that may be suppurative, with frequent
adrenal gland, CNS, and bone involvement. Men comprise more than
90% of those afflicted with this form of paracoccidioidomycosis.
A proposed reason for this is that form development from the
mold to the yeast form is inhibited by estrogen (Shankar et al,
2011).
Oropharyngeal lesions are the result of dissemination from a
pulmonary focus of infection affecting the mucous membranes of
the mouth, loosening teeth resulting in their loss. White
plaques are found in the buccal mucosae, and this along with the
triad are now used to clinically differentiate between TB and
paracoccidioidomycosis. Other sites of dissemination include
skin and internal organs. An important feature of the
disease is a long latent period. Ten to twenty years may pass between infection and
emergence of the disease, sometimes in non-endemic areas of the
world.
Therapy
Itraconazole and trimethoprim-sulfamethoxazole are the
choices for primary therapy with similar effectiveness. The time
to reach clinical cure is shorter with itraconazole, including
those patients with the chronic clinical form.
Laboratory
Specimens to send to the lab: Sputum, lower respiratory tract
secretions, bone marrow, biopsy material from liver or spleen,
pus and crust from lesions on skin and mucous membranes, pus
from draining lymph nodes.
Direct microscopic examination: Microscopic exam of sputum or
crust from a lesions is done by first adding a drop of 10% KOH.
Identification of P. brasiliensis in sputum is more difficult
than in skin scrapings and lymph node specimens. When sputum is
clarified with 10% KOH and then homogenized yields are higher
than only using cleared sputum.
Tissue specimens may be cut with a razor blade or homogenized
and examined microscopically before seeding to culture medium.
Microscopy reveals the yeast form which, in contrast to other
fungal pathogens, displays multipolar budding, a thin cell wall,
and a narrow base bud: the so-called “mariner’s wheel”. Yeast
forms vary from 2-30 µm diam. Strains also vary in that the
mother cell may be similar in size to the daughter cells or much
larger.
Culture
P. brasiliensis mold form is cultured on Mycosel or
Mycobiotic Agar, SABHI (Difco), Sabouraud agar or yeast extract
agar. Medium with cycloheximide is permissive for mold form
growth but inhibits the yeast form. Sputum samples are
pre-digested with pancreatin or N-acetyl-L-cysteine. Incubation
is at room temperature. Growth of the mold form is very slow and
may take 15-20 d before growth is visible.
At 25 degrees C, the colony is a dense, white mycelium not loose and
cottony .On Sabouraud dextrose agar it may take 2-3 wks to grow.
Note: the Blastomyces Accuprobe®, Hologics, Inc. gives a
positive reaction with P. brasiliensis and may be used in the
diagnostic work-up.
Since sporulation is scarce, conversion to the yeast form is an
important part of the lab diagnosis. This is done on agar slants
containing brain-heart infusion or Kelley agars, incubated at
35-36ºC. The yeast form is also slow growing into a white-tan,
thick colony.
Histopathology
Biopsied tissue specimens are formalin-fixed,
paraffin-embedded, then stained with either or both H&E and Gomori methenamine silver. H&E staining shows the host
inflammatory response, granuloma formation, and Paracoccidioides
sp. yeast forms (fig.21). GMS stains the fungus wall showing the
typical yeast forms with multipolar buds, but not the
inflammatory response (fig. 22).
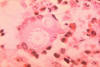 Fig. 21. Histopathology of a skin tissue sample in a case of
paracoccidioidomycosis. A budding yeast form of Paracoccidioides
brasiliensis is phagocytosed by a multinucleate giant cell.
Fig. 21. Histopathology of a skin tissue sample in a case of
paracoccidioidomycosis. A budding yeast form of Paracoccidioides
brasiliensis is phagocytosed by a multinucleate giant cell.
Source.
Image #522 Dr. Lucille K. George, CDC Public Health Image Library.
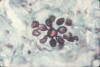 Fig. 22. Photomicrograph of a Gomori methenamine silver stained
unidentified tissue sample, shows the yeast form of Paracoccidioides
brasiliensis, in a case of paracoccidioidomycosis. The multipolar
budding yeast form resembles what has been referred to as mariner’s
wheel.
Fig. 22. Photomicrograph of a Gomori methenamine silver stained
unidentified tissue sample, shows the yeast form of Paracoccidioides
brasiliensis, in a case of paracoccidioidomycosis. The multipolar
budding yeast form resembles what has been referred to as mariner’s
wheel.
Source: #498 CDC Public Health Image Library
Serology
Detection of antibodies by agar gel diffusion is the
main test for diagnosis. Accuracy is high and results correlate
with response to therapy. Antigens used are (1) yeast-phase cell
culture filtrate antigen and (2) supernatant from sonicated cell
suspensions. Sensitivity is estimated to be 77%, and
specificity, 95%.
Endpoint serum dilution titers indicate severity of disease and
subside in response to therapy. P. brasiliensis 43-kDa
glycoprotein (gp43) is significant in the immune response in PCM
but some P. brasiliensis isolates do not express gp43.The
identification of different species - P. brasiliensis and
P. lutzii and P. brasiliensis cryptic species suggests that
antigens be prepared from the dominant species in a particular
region.
ELISA can detect very low antibody concentrations with
sensitivity and specificity depending on the antigen used and
the selected cut-off point. Using P. brasiliensis yeast form
culture filtrate antigens and a titer of 1:80 as the cut-off
point for a positive test, sensitivity and specificity of ELISA
reached 100%. Cross-reactions between carbohydrate epitopes in
the crude antigen mix are seen with serum from patients with
histoplasmosis, cryptococcosis, aspergillosis, and lacaziosis (lobomycosis).
Specific antibodies from PCM patients react predominantly with
gp43 peptide epitopes. Specificity can be improved by sodium
metaperiodate treatment, which inactivates carbohydrates i.e.:
galactomannan.
SPOROTRICHOSIS (Sporothrix schenckii)
Introduction
Sporotrichosis is a pyogranulomatous disease
affecting the cutaneous and subcutaneous tissues caused by a
puncture with a thorn or splinter carrying conidia of the
dimorphic mold, Sporothrix schenckii. Also known as "rose
gardener’s disease." It is world-wide in distribution with areas
of high endemicity.
Disease Definition
Sporotrichosis is a chronic cutaneous or
subcutaneous pyogranulomatous infection. Three clinical forms
are fixed cutaneous, lymphocutaneous and, less commonly,
extracutaneous. The causative agent is the dimorphic fungus, Sporothrix schenckii. Sporotrichosis is acquired through
recreational or occupational exposure by gardeners or workers
handling plants or sphagnum moss, or by scratches from an
infected domestic cat. A puncture from a thorn or splinter on an
extremity (usually on the hand) implants conidia and a pustule
develops (fig 23, a thorn prick on the thumb). It may remain
fixed and heals with scarring or spreads via a chain of
subcutaneous nodules tracing the path of lymphatic drainage from
the primary lesion. Progression usually stops at the axilla. The
nodules suppurate, drain, and ulcerate. (figs 24, 25). The less
common extracutaneous clinical form may occur by penetrating
trauma into a joint or the eye, or via dissemination from the
skin or lungs. Pulmonary sporotrichosis is likely acquired via
inhalation of conidia from the environment.
 Fig. 23. Anterior view of patient’s left thumb, with a swelling in
the distal interphalangeal joint, due to a fungal sporotrichosis.
Fig. 23. Anterior view of patient’s left thumb, with a swelling in
the distal interphalangeal joint, due to a fungal sporotrichosis.
Source. #3011, CDC Public Health Image Library, Dr. William Kaplan
 Fig. 24. Sporotrichosis, Primary lesion on hand.
Fig. 24. Sporotrichosis, Primary lesion on hand.
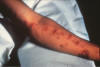 Fig . 25 Lymphocutaneous sporotrichosis (see text for explanation)
Fig . 25 Lymphocutaneous sporotrichosis (see text for explanation)
Source: Dr. H.J. Shadomy
Diagnosis
Tissue biopsy, scrapings, or swabs of exudates are
sent to the laboratory for culture and identification, as the
most direct approach to diagnosis. Only the yeast form of Sporothrix schenckii
is present in lesions whereas the mold form
usually grows rapidly (5-6 d) on fungal medium, (e.g.: Emmons’
modified Sabouraud Dextrose agar). See “Laboratory” for growth
characteristics.
Differential diagnosis
Sporotrichosis is clinically similar to
other causes of nodular lymphangitis: nocardiosis, Mycobacterium marinum,
M. chelonae, tularemia, and leishmaniasis.
Etiologic Agents
The classic agent, S. schenckii, is part of a
complex of phylogenetically related species divided into a
clinical clade (mostly human pathogens) including S. brasiliensis,
S. schenckii, S. globosa, and S. luriei, and an
environmental clade.
Geographic Distribution/Ecologic Niche
Sporotrichosis occurs
worldwide with highly endemic sub-regions in the Americas and in
Asia:
- Central highlands of Mexico
- Southern Andean mountains
- Rio de Janeiro, Brazil--a zoonotic outbreak of sporotrichosis
transmitted by domestic cats.
- Northeast China, India, eastern coast of Kyushu, Japan
(Orofino-Costa et al, 2017).
The ecologic niche for this organism is rose thorns, sphagnum
moss, timbers and soil.
Epidemiology
Incidence and Prevalence Sporotrichosis was thoroughly
investigated during an epidemic of 3,000 gold mine workers who
brushed against timbers in the mine in South Africa during the
1940's. A number of outbreaks were related to handling sphagnum
moss.
Rio de Janeiro, Brazil is hyperendemic for cat-associated
sporotrichosis (Gremião et al 2017). From 1997 to 2011, 4,188
human cases were recorded in Rio de Janeiro. Since 1998, 244
dogs were diagnosed through 2014, and 4,703 cats were diagnosed
through 2015. Unlike classic transmission due to conidia,
zoonotic transmission is with yeast cells of S. brasiliensis via
cat scratch or bite.
Risk Groups/Factors
Occupational distribution of sporotrichosis. Forest employees
accounted for 17% of cases, gardeners and florists, 10%; other
soil-related occupations 16%. Poor socioeconomic conditions in
the Rio de Janeiro area are risk factors for zoonotic (cat to
human) transmission.
Transmission
Puncture wounds from thorn or splinter initiates
infection. Inhalation of conidia is an uncommon route. Cat-human
transmission is addressed above.
Determinants of Pathogenicity
(Téllez et al., 2014)
Growth at
37oC is a requirement for pathogenicity, as it is for other
dimorphic fungal pathogens. Mold to yeast dimorphism is another
pathogenic factor. Cell functions that protect the fungus in the
environment also are implicated in host pathogenicity: Melanin
resists phagocytosis and oxidative killing by host phagocytes;
adherence to dermal and connective tissue matrix facilitates
invasion; proteinase can damage host tissue. Catalase,
superoxide dismutase, and nitroreductase all protect the fungus
from oxidative fungicidal mechanisms of host phagocytes.
Clinical Forms
Fixed cutaneous
Single or a few lesions appear without
lymphatic spread. They appear nodular, verrucous, or ulcerative
and may resemble squamous cell carcinoma.
Lymphocutaneous
After a puncture wound with a thorn or
splinter, usually on an extremity, a small nodule appears and
may ulcerate. Secondary subcutaneous lymphatic nodules appear in
a linear chain extending along the path of lymphatic drainage.
They may ulcerate and drain.
Extracutaneous
The osteoarticular form may occur by extension
from the skin or via hematogenous spread. In recent years,
pulmonary disease has been seen more frequently. Occasionally,
infection with S. schenckii may result in a mycetoma. Rarely,
inhalation of conidia results in fungal pneumonia.
Veterinary Forms
Epizootic feline sporotrichosis emerged among
feral cats in Rio de Janeiro state (Gremião et al 2015).
Affected animals have multiple skin lesions with mucosal
involvement of the respiratory tract. Skin lesions occur in cats
as nodules and ulcers found at three or more sites, the head,
nose accompanied by lymphangitis. Also present are respiratory
signs of sneezing, dyspnea, nasal discharge and mucosal
involvement .Severe pyogranulomatous inflammatory infiltrate,
high fungal load, and extension of lesions to mucosae,
cartilage, and bone in the nose of cats underline this is a
virulent agent in the endemic region. The drug of choice is
itraconazole, also including other options such as iodides,
ketoconazole, terbinafine, local heat therapy, and surgical
excision. High cost, lack of compliance, and difficulty of oral
administration to cats remain obstacles to success.
Therapy. Lymphocutaneous form: saturated solution of potassium
iodide (oral) or itraconazole. Extracutaneous: itraconazole or
amphotericin B.
Laboratory
Clinical material to be sent to the lab: pus, biopsy
material, or sputum.
Direct examination and histopathology are hindered by the
paucity of yeast forms in the lesions.
Direct examination. (10% KOH mount) Observe for budding yeast
which are small round or fusiform (cigar-shaped) and 2 to 6 µm
diam. They are scarce and difficult to detect. Fusiform yeast
forms are usually diagnostic for sporotrichosis (figs 26, 27)
Yeast forms of S. schenckii may be confused with those of
H. capsulatum and Candida glabrata. When no KOH is used, asteroid
bodies may be observed by direct exam.
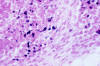 Fig. 26 Histopathology of sporotrichosis, shows fusiform (cigar
shaped) yeast forms, usually diagnostic for sporotrichosis.
Fig. 26 Histopathology of sporotrichosis, shows fusiform (cigar
shaped) yeast forms, usually diagnostic for sporotrichosis.
Source:
Dr. Arthur F. DiSalvo
 Fig. 27 Sporothrix schenckii yeast forms in culture. Note the
fusiform shape which is diagnostic. 970x magnification.
Fig. 27 Sporothrix schenckii yeast forms in culture. Note the
fusiform shape which is diagnostic. 970x magnification.
Source: CDC
Public Health Image Library # 22003, Dr. Lucille K. Georg
Histopathology
S. schenckii
yeast forms may be scarce in tissue
sections. Yeasts are not easy to detect with H&E stains so GMS
or PAS stains are preferred (fig 26). Eosinophilic material
surrounds many yeast forms in tissue. These are the “asteroid
bodies” (also called Splendore- Hoeppli material) which are
distinctive features of sporotrichosis. In the background there
is granulomatous inflammation also including neutrophils and
microabscesses (fig 28).
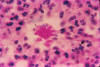 Fig. 28 Asteroid body, sporotrichosis (H & E stain).
Fig. 28 Asteroid body, sporotrichosis (H & E stain).
Source: Dr.
H.L. Lurie
Mycology
Growth on media is rapid, in 5-6 d.
Mold form
Colony morphology. At 25
degrees C, the colony is white-cream and
membranous (fig. 29). As it ages (2-3 wks) it becomes black and
leathery (fig. 30).
Microscopic morphology. The mycelium is branching, septate and
very delicate, 2-3 µm diam (At the end of threadlike
conidiophores pyriform conidia, 2-4 um diam, form a "daisy
cluster" (fig. 31). With aging, a sleeve of melanized conidia
develops along the hyphae (fig. 32).
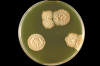 Fig. 29. Mold form of Sporothrix schenckii, young culture.
Fig. 29. Mold form of Sporothrix schenckii, young culture.
Source: Dr. William Kaplan, CDC
 Fig. 30. Older mold culture of Sporothrix schenckii showing
dark coloration due to melanized conidia. These are the sessile
conidia that appear as a sheath along the growing hyphae.
Fig. 30. Older mold culture of Sporothrix schenckii showing
dark coloration due to melanized conidia. These are the sessile
conidia that appear as a sheath along the growing hyphae.
Source: Indiana Pathology Associates M406-66
 Fig. 31. Sporothrix schenckii mold form, slide culture shows
conidiophore with florets of conidia on slender denticles.
Fig. 31. Sporothrix schenckii mold form, slide culture shows
conidiophore with florets of conidia on slender denticles.
Source: Dr. E. Reiss
 Fig 32. Sporothrix schenckii mold form, slide culture. This
photo depicts a sleeve of sessile conidia borne directly on hyphae
(conidia are melanized).
Fig 32. Sporothrix schenckii mold form, slide culture. This
photo depicts a sleeve of sessile conidia borne directly on hyphae
(conidia are melanized).
Source: Dr. E. Reiss
Yeast form
Mold to yeast conversion occurs rapidly on blood-enriched media
at 35-37 oC.
Colony morphology. Creamy smooth, tan, yeast-like.
Microscopic morphology. Elongate, budding cells, cigar-shaped,
some round or oval.
Talaromycosis (formerly Penicilliosis) Talaromyces marneffei
Introduction
Talaromycosis is an endemic mycosis geographically
restricted to SE Asia, especially Thailand, caused by the
dimorphic fungus Talaromyces marneffei. Unlike disease caused by
other endemic dimorphic fungi T. marneffei is an opportunistic
pathogen closely, but not entirely, associated with the AIDS
pandemic.
Disease
Definition
Talaromycosis is initiated by inhalation of
conidia of Talaromyces marneffei from the environment by an
immunocompromised host, (e.g.: HIV-positive). The fungus
undergoes dimorphic transition in the lungs to a fission yeast
and is spread hematogenously, especially to the skin, but also
to other internal organs.
Diagnosis
Direct examination and culture of scrapings, biopsy
or exudate from skin lesions, from blood samples. The infectious
form is a fission yeast. On mycologic media at below 30oC the
mold form grows rapidly and secretes a red pigment.
Etiologic Agents
Talaromyces marneffei is classified as:
Ascomycota, Pezizomycotina, Eurotiomycetes, Eurotiomycetidae,
Eurotiales, Trichocomaceae, Talaromyces (Tsang et al., 2018)
Geographic
Distribution/Ecologic Niche
Tropical and sub-tropical climates of southern China, Thailand
and other parts of SE Asia. The natural source for T. marneffei
is the bamboo rat (Rhizomys sinensis, and related spp.). Bamboo
rats may act as reservoirs, but direct rat to human transmission
is unknown. A hyperendemic area is Chiang Mai province in
Thailand. Despite efforts, T. marneffei has not, to date, been
isolated from the environment.
Epidemiology
All cases are from persons living in or traveling
to the SE Asia endemic area: Thailand, Vietnam, Hong Kong,
southern China, Taiwan, India, Indonesia, Cambodia and Laos. 10%
of AIDS patients in Hong Kong and ~30% of patients in N.
Thailand present with Talaromyces marneffei infections. Patients
with AIDS, or other immunosuppressive conditions, and
talaromycosis are diagnosed in other countries after travel or
residence in the
endemic area.
Risk Groups/Factors
Residents of or travelers to the endemic
area who have compromised immune systems are susceptible to talaromycosis. It is an AIDS-defining opportunistic infection
affecting all ages and sexes but a preponderance of cases are
male. Untreated talaromycosis has high mortality in HIV-infected
patients. Infections also occur in immunocompromised patients
who are not infected with HIV. Those Immunosuppressive
conditions include autoimmune disease, solid organ or
hematopoietic stem cell transplants, or other
T-lymphocyte-depleting immunosuppressive therapy. Other persons
at increased risk are those with anti-IFN-γ autoantibodies, or
hematologic malignancy (Chan et al., 2016). HIV-negative persons
who are not immunosuppressed are also at risk including those
with diabetes (Qiu et al, 2015).
Transmission
Infection is initiated by inhalation of conidia in
the endemic area, human to human transmission is exceptionally
rare.
Determinants of Pathogenicity
The ability of this mold to
undergo morphogenesis to the fission yeast form is clearly
related to its pathogenicity. A molecule identified by genomic
means, Mp1p, is a cell wall and secreted antigenic mannoprotein
(Lau et al., 2017). Mp1p may be useful for serologic diagnosis
of T. marneffei. The virulence property of Mp1p is thought to
aid in evasion of host defense by improving survival within host
macrophages.
Clinical Forms
In HIV-infected patients,
T. marneffei infection
is often disseminated involving multiple organs. In
non-HIV-infected patients the infection may be focal or
disseminated depending on the extent of underlying
immunocompromise, and the delay in diagnosis.
Disseminated talaromycosis
Fever, fatigue, wt loss, lymphadenopathy, hepatosplenomegaly, umbilicated skin lesions,
primarily on the face or as diffuse cutaneous nodules (fig. 33). Skin lesions are the result of hematogenous dissemination.
Disease progresses to multiple organ involvement, respiratory
failure, and shock. Pulmonary involvement is seen as
interstitial to alveolar infiltrates, reticulonodular
consolidation and, occasionally, a miliary pattern similar to
tuberculosis (Salzer et al, 2018).
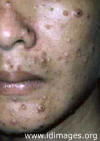 Fig. 33 Talarormycosis, skin lesions on face.
Fig. 33 Talarormycosis, skin lesions on face.
Case #03068: A young man with a fever and facial rash.
Source:
Copyright Partners Healthcare System, Inc. All rights reserved.
Veterinary Forms
Four species of bamboo rats, Rhizomys sinensis,
R. pruinosus, R. sumatrensis, and Cannomys badius, are natural
hosts of T. marneffei. Trapped animals may appear healthy but
T. marneffei could be isolated from their spleens (Gugnani et al.,
2004). Isolation of T. marneffei from soil has not been
reported.
Therapy
First line therapy consist of liposomal amphotericin B,
3–5 mg/kg/d IV for 1–2 wks followed by itraconazole for 6-12-wks
(Salzer et al., 2018). AmB deoxycholate is a second choice, also
followed by step down to itraconazole.
Laboratory
Direct Examination
Specimens submitted to the lab include skin
scrapings, blood (for smear and culture).
Culture. Specimens include: blood, sputum, lymph node, lung
tissue, bone, bone marrow. Plate to blood agar or SDA at 25o and
37oC and incubate for 1 wk. Growth will also occur in blood
culture bottles.
Mold form
Colony morphology. At 25
degrees, C T. marneffei is a fast growing,
suede-like to downy, white mold with yellowish-green conidial
heads. Colonies become grey-pink to brown and secrete a
diffusible brown-red to wine-red pigment (fig 34).

 Fig. 34 Colony morphology Talaromyces marneffei (a) surface, (b)
reverse.
Fig. 34 Colony morphology Talaromyces marneffei (a) surface, (b)
reverse.
Source: Jim Gathany, CDC
Microscopic morphology
Hyphae are hyaline. Conidiophores
biverticillate, sometimes monoverticillate.
Terminal verticils of 3 to 5 metulae, each with 3-7 flask shaped
phialides. Conidia are 2-3 μm
diam. produced in basipetal formation from phialides (fig.35).
 Fig. 35. Microscopic morphology Talaromyces marneffei mold
form.
Fig. 35. Microscopic morphology Talaromyces marneffei mold
form.
Source: Dr. E. Reiss
Fission yeast form
Colony morphology. Growth on brain heart infusion+ RBC agar at
37OC, colonies are yeastlike, tan color. Microscopic morphology.
Yeast cells round to ellipsoid (2-6 µm diam) and divide by
fission, hyphal fragments present,
Red pigment. P. marneffei secretes a diffusible red pigment when
grown below 30 °C, a feature important for its identification
(Tam et al., 2015). The red pigment consists of monascorubrin
and rubropunctatin.
Histopathology. Tissue sections stained with periodic
acid-Schiff, or Wright’s stain and examine for fission yeast
within and outside of macrophages (fig.36).
 Fig. 36 Histopathology Talaromyces marneffei. Arrows indicate
division by fission.
Fig. 36 Histopathology Talaromyces marneffei. Arrows indicate
division by fission.
Source: Benjaporn Chaiwun, M.D., Department of
Pathology, Faculty of Medicine, Chiang Mai University, Chiang Mai,
Thailand.
Some taxonomic definitions appropriate for medical
mycology (de Hoog et al., 2015)
Species complex
A monophyletic clade of species with equivalent
clinical relevance
Sibling species
Species that share the same, most recent common
ancestor
Cryptic species
Species recognized by nucleic acid variation
that had not been recognized as distinct by morphologic
phenotypes. Once recognized, phenotypic characters useful for
identification may be discovered in the future.
(Sub) clade/monophyletic group
Phylogenetic group consisting of
an ancestral species and all its descendants. Clades and
subclades can be recognized at any taxonomic level. Statistical
tests are used to gauge the support for these groups.
Lineage
Series of species connected by evolutionary descent, not
necessarily representing all known descendants
Cluster/group
Terminal series of phylogenetically related
species, used when precise relationships are uncertain.
Type
Entity defining a taxonomic name and indicated as such in
the protologue. Species and below are defined by a specimen,
whereas higher taxonomic entities are defined by the first lower
category.
Neotype
New specimen in accordance with the protologue in case
the original type material is lost.
Epitype
Reference specimen accordance with the protologue when
the original material is not interpretable.
Protologue
Original description and any other representation of
a taxonomic entity.
|






































