|
x |
x |
|
 |
 |
|
INFECTIOUS
DISEASE |
BACTERIOLOGY |
IMMUNOLOGY |
MYCOLOGY |
PARASITOLOGY |
VIROLOGY |
|
|
VIROLOGY - CHAPTER TWELVE
VIRUS-HOST INTERACTIONS
Dr Gene Mayer
Emeritus Professor
Department of Pathology, Microbiology and Immunology
University of South Carolina School of Medicine
Columbia
|
|
En
Español |
|
|
Let us know what you think
FEEDBACK |
|
SEARCH |
|
|

 |
|
Logo image
© Jeffrey Nelson, Rush University, Chicago, Illinois and
The MicrobeLibrary |
|
|
|
|
|
TEACHING
OBJECTIVES
To
describe host specific and nonspecific defense mechanisms involved in
resistance to and recovery from virus infections
To
discuss the role of interferon in viral infections
To
review the mechanisms by which interferon exerts its antiviral activity
To
discuss the relative contributions of various host defense mechanisms in
viral infections |
Resistance to and recovery from
viral infections will depend on the interactions that occur between virus and
host. The defenses mounted by the host may act directly on the virus or
indirectly on virus replication by altering or killing the infected cell. The
non-specific host defenses function early in the encounter with virus to prevent
or limit infection while the specific host defenses function after infection in
recovery immunity to subsequent challenges. Although the host defense mechanisms
involved in a particular viral infection will vary depending on the virus, dose
and portal of entry, some general principals of virus-host interactions are
summarized below.
BARRIERS TO INFECTION
Inherent Barriers
The host
has a number of barriers to infection that are inherent to the organism.
These represent the first line of defense which function to prevent or
limit infection.
Skin
The skin
acts a formidable barrier to most viruses and only after this barrier is
breached will viruses be able to infect the host.
Lack of Membrane
Receptors
Viruses gain entry into host cells by first binding to
specific receptors on cells (Table 1; adapted from: Roitt, Immunology, 5th
Ed).
|
KEY
WORDS
Inherent
defenses
Induced
defenses
Interferon
2'5'
Oligo A synthetase
IFN-activated
protein kinase
Intrinsic
antiviral activity
Extrinsic
antiviral activity
ADCC
Immune
adherence
NK cells
CTLs |
|
Table 1 |
|
Virus |
Receptor |
Cell Type Infected |
|
HIV |
CD4 |
TH cells |
|
Epstein-Barr virus |
CR2 (complement receptor type 2) |
B cells |
|
Influenza A |
Glycophorin A |
Many cell types |
|
Rhino virus |
ICAM-1 |
Many cell types |
The host range of the virus
will depend upon the presence these receptors. Thus, if a host lacks the
receptor for a virus or if the host cells lacks some component necessary
for the replication of a virus, the host will inherently be resistant to
that virus. For example, mice lack
receptors for polio viruses and thus are resistant to polio virus.
Similarly, humans are inherently resistant to plant and many animal
viruses.
Mucus
The mucus
covering an epithelium acts as a barrier to prevent infection of host cells.
In some instances the mucus simply acts as a barrier but in other cases
the mucus can prevent infection by competing with virus receptors on
cells. For example, orthomyxo- and paramyxovirus families infect the host cells
by binding to sialic acid receptors. Sialic acid-containing
glycoproteins in mucus can thus compete with the cell receptors and
diminish or prevent binding of virus to the cells.
Ciliated epithelium
The ciliated epithelium which drives the mucociliary elevator can help
diminish infectivity of certain viruses. This system has been shown to be
important in respiratory infections since, when the activity of this system
is inhibited by drugs or infection, there is an increased infection rate
with a given inoculum of virus.
Low pH
The low pH
of gastric secretions inactivate most viruses. However, enteroviruses are
resistant to gastric secretions and thus can survive and replicate in the
gut.
Humoral and cellular
components
See below
|
| |
Induced Barriers
Changes
that occur in the host in response to infection can also help diminish
virus infectivity.
Fever
Fever can
help to inhibit virus replication by potentiating other immune defenses
and by decreasing virus replication. The replication of some viruses is
reduced at temperatures above 37degrees C.
Low pH
The pH of
inflammatory infiltrates is also low and can help limit viral infections
by inactivating viruses.
Humoral and cellular
components
See below
HUMORAL COMPONENTS INVOLVED
IN RESISTANCE TO VIRAL INFECTIONS
Nonspecific
A
number of humoral components of the nonspecific immune system function in
resistance to viral infection. Some of theses are constitutively present
while others are induced by infection.
Interferon (IFN)
IFN was discovered over 40 years ago by Issacs and Lindemann who showed
that supernatant fractions from virus-infected cells contained a protein that could
confer resistance to infection to other cells. This substance did not act
directly on the virus, rather it acted on the cells to make them resistant
to infection (Figure 1).
|
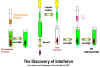 Fig. 1. The discovery of interferon
Fig. 1. The discovery of interferon
 Fig. 2. Typical response to an acute virus infection
Fig. 2. Typical response to an acute virus infection |
IFN is one of the first lines
of defense against viruses because it is induced early after virus
infection before any of the other defense mechanisms appear (e.g.
antibody, Tc cells etc.) (Figure 2). The time after which IFN
begins to be made will vary depending on the dose of virus.
a) Types and
Properties of Interferons
Table 2; Adapted from: Murray,
Medical Microbiology, 5th Ed. Table 14-3)
|
Table 2
Types and
Properties of Interferon |
|
|
Interferon |
|
Property |
Alpha |
Beta |
Gamma |
|
Previous designations |
Leukocyte IFN
Type I |
Fibroblast IFN
Type I |
Immune IFN
Type II |
|
Genes |
>20 |
1 |
1 |
|
pH2 stability |
Stable |
Stable |
Labile |
|
Inducers |
Viruses (RNA>DNA)
dsRNA |
Viruses(RNA>DNA)
dsRNA |
Antigens, Mitogens |
|
Principal source |
Leukocytes, Epithelium |
Fibroblasts |
Lymphocytes |
There are three types of
interferon, IFN-alpha (also known as leukocyte interferon), IFN-beta
(also known as fibroblast interferon) and IFN-gamma (also known as
immune interferon). IFN-alpha and IFN-beta are also referred to as
Type I interferon and IFN-gamma as Type II. There are
approximately 20 subtypes of IFN-alpha but only one IFN-beta and IFN-gamma.
The interferons have
different characteristics that could be used to distinguish them (e.g.
pH stability and activity in the presence of SDS) but currently they are
identified by using specific antibodies to the interferons.
b) Inducers of
Interferons - Normal cells do not contain preformed IFN nor do
they secret interferon constitutively. This is because the interferon
genes are not transcribed in normal cells. Transcription of the IFN
genes occurs only after exposure of cells to an appropriate inducer.
Inducers of IFN-alpha and IFN-beta include virus infection, double
stranded RNA (e.g. poly inosinic:poly cytidylic acid; [poly I:C]),
LPS, and components from some bacteria. Among the viruses, the RNA
viruses are the best inducers while DNA viruses are poor IFN inducers,
with the exception of poxviruses. Inducers of IFN-gamma include
mitogens and antigen (i.e. things that activate lymphocytes).
|
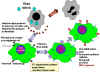 Fig. 3. Mode of action of interferon
Fig. 3. Mode of action of interferon |
c) Cellular Events in
the Induction of Interferons
The IFN genes are not expressed
in normal cells because the cells produce a labile repressor protein
that binds to the promoter region upstream of the gene and inhibits
transcription. In addition, transcription of the genes require activator
proteins to bind to the promoter region and turn on transcription.
Inducers of IFN act by either preventing synthesis of the repressor
protein or increasing the levels of the activator proteins, thereby
turning the IFN gene on. After the inducer is gone, the IFN gene is
again turned off by the repressor protein and/or the lack of activator
proteins. Once the gene is turned on, it is transcribed, the mRNA is
translated and the protein is secreted from the cell. The IFN will bind
to IFN-receptors on neighboring cells and induce an antiviral state in
the second cell (Figure 3)
|
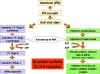 Fig. 4 Molecular basis of the antiviral state
Fig. 4 Molecular basis of the antiviral state |
d) Cellular Events in
the Action of Interferons - The binding of IFN to its receptor
results in the transcription of a group of genes that code for antiviral
proteins involved in preventing viral replication in that cell. As a
consequence the cell will be protected from infection with a virus until
the antiviral proteins are degraded, a process which takes several days.
The antiviral state in IFN-treated cells results from the synthesis of
two enzymes that result in the inhibition of protein synthesis. One
protein indirectly affects protein synthesis by breaking down viral mRNA
the other directly affects protein synthesis by inhibiting elongation (Figure
4).
One protein, called
2'5'Oligo A synthetase, is an enzyme that converts ATP into a unique
polymer (2'5' Oligo A) containing 2'- 5'phophodiester bonds.
Double stranded RNA is required for the activity of this enzyme. The
2'5'Oligo A in turn activates RNAse L which then breaks down viral mRNA.
The second protein is an protein kinase that, in the presence of double
stranded RNA, is autophosphorylated and thereby activated. The activated
protein kinase in turn phosphorylates elongation factor eIF-2 and
inactivates it. By the action of these two IFN-induced enzymes protein
synthesis is inhibited. Although the infected cell may die as a
consequence of the inhibition of host protein synthesis, the progress of
the infection is stopped. Uninfected cells are not killed by IFN
treatment since activation of the two enzymes requires double stranded
RNA, which is not produced. Some viruses have means of inhibiting the
antiviral effects of IFN. For example the adenoviruses produce an RNA
which prevents the activation of the protein kinase by double stranded
RNA thereby reducing the antiviral effects of IFN.
|
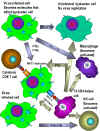 Fig. 5 Effects of interferons alpha, beta and gamma
Fig. 5 Effects of interferons alpha, beta and gamma |
e) Other Biological
Activities of Interferons - IFN not only induces the production
of antiviral proteins, it also has other effects on cells, some of which
indirectly contribute to the ability of the host to resist or recover
from a viral infection (Figure 5). IFN can help modulate immune responses by its
effects on Class I and Class II MHC molecules. IFN-alpha, IFN-beta
and IFN-gamma increase expression of Class I molecules on all cells
thereby promoting recognition by Tc cells which can destroy virus
infected cells. IFN-gamma can also increase expression of Class II MHC
molecules on antigen presenting cells resulting in better presentation
of viral antigens to CD4+ T helper cells. Furthermore, IFN-gamma
can activate NK cells which can kill virus infected cells. IFNs also
activate the intrinsic and extrinsic antiviral activities of
macrophages. Intrinsic antiviral activity is the ability of macrophages
to resist infection with a virus and extrinsic antiviral activity is the
ability of macrophages to kill other cells infected with virus. The IFNs
also have anti-proliferative activity making them useful in the
treatment of some malignancies.
|
| |
f) Clinical Uses of
Interferons - IFNs have been used in the treatment of a number
of viral and other diseases (Table 3; Adapted from: Mims, Medical
Microbiology, Fig 37.5 )
|
Table 3
Clinical Uses of Interferons |
|
Interferon |
Therapeutic use |
|
IFN-alpha, IFN-beta |
Hepatitis B (chronic)
Hepatitis C
Herpes zoster
Papilloma virus
Rhino virus (prophylactic only)
Warts |
|
IFN-gamma |
Lepromatous leprosy
Leshmaniasis
Toxoplasmosis
Chronic granulomatous disease (CGD) |
|
| |
In addition due to its anti
proliferative effects IFNs have also been used in the treatment of a
variety of cancers (Table 4; Adapted from: Zinsser, Microbiology, 20th
Ed, Table 58.3).
|
Table 4
Use of Interferons on Cancer
Treatment |
|
Tumor |
Percent Complete or Partial
Remissions |
|
Hairy cell leukemia |
90 |
|
Chronic myelocytic leukemia |
90 |
|
T cell lymphoma |
53 |
|
Kaposi's sarcoma |
42 |
|
Endocrine pancreatic neoplasms |
30 |
|
Non-Hodgkin's lymphomas |
25 - 35 |
|
| |
However, the side effects
of IFN therapy limits their casual use in clinical medicine (Table 5;
Adapted from: Mims, Medical Microbiology, Fig. 37.6).
|
Table 5
Common Side Effects
of Interferons |
|
Interferons |
Fever
Malaise
Fatigue
Muscle pains
Toxicity to:
kidney
liver
bone marrow
heart
|
|
| |
Complement
Most
viruses do not fix complement by the alternative route. However, the
interaction of a complement-fixing antibody with a virus infected cell or
with an enveloped virus can result in the lysis of the cell or virus.
Thus, by interfacing with the specific immune system, complement also
plays a role in resistance to viral infections.
Cytokines
Cytokines other than IFN also may play a role in resistance to virus
infection. Tumor necrosis factor alpha (TNF-α), interleukin-1 (IL-1)
and IL-6 have been shown to have antiviral activities in vitro.
These cytokines are produced by activated macrophages but their
contribution to resistance in vivo has not been fully elucidated.
|
| |
Specific
Antibody
produce by the specific immune system is involved primarily in the
recovery from viral infection and in resistance to subsequent challenge
with the virus. IgG, IgM and IgA antibodies can all play a role in
immunity to virus infection but the relative contributions of the
different classes depends on the virus and the portal of entry. For
example, IgA will be more important in viruses that infect the mucosa
while IgG antibodies will be more important in infections in which viremia
is a prominent feature. Antibodies can have both beneficial and harmful
effects for the host.
Beneficial effects
(Table 6; Adapted from: Roitt, Immunology 5th Ed., Fig 16.5)
Antibody can directly neutralize virus infectivity by preventing the
attachment of virus to receptors on host cells or entry of the virus into
the cell. Antibodies can also prevent uncoating of virus by interfering
with the interaction of viral proteins involved in uncoating. Complement
fixing antibodies can assist in the lysis of viral infected cells or
enveloped viruses. Antibodies can also act as an opsonins and augment
phagocytosis of viruses either by promoting their uptake via Fc or C3b
receptors or by agglutinating viruses to make them more easily
phagocytosed. Antibody coated virus infected cells can be killed by K
cells thereby preventing the spread of the infection.
|
Table 6
Antiviral Effects of Antibody |
|
Target |
Agent |
Mechanism |
|
Free virus |
Antibody alone |
Blocks binding to cell
Blocks entry into cell
Blocks uncoating of virus |
|
Antibody + Complement |
Damage to virus envelope
Opsonization of virus |
|
Virus-infected cell |
Antibody + Complement |
Lysis of infected cell
Opsonization of infected cell |
|
Antibody Bound to Infected Cells |
ADCC by K cells, NK cells and/or
macrophages |
Harmful
effects
-
Immunopathological
damage
Fixation of complement by immune complexes can result
in the release of vasoactive amines, recruitment of inflammatory cells
and subsequent damage to host tissue. Some viruses such a lymphocytic
choriomeningitis virus produce large amounts of immune complexes in the
circulation which lodge in the vascular beds and in the kidneys where
they fix complement and result in tissue damage. Other examples of
viruses that cause these effects are: measles, respiratory syncytial
virus, dengue and serum hepatitis virus.
-
Immune adherence
Opsonization of viruses with antibody can enhance their uptake by
phagocytic cells. If the virus is able to survive in the phagocyte, this
allows for the spread of virus infection. Dengue and HIV are examples of
viruses that can survive in macrophages.
Serology
Since
the isolation and identification of viruses is not commonly done in the
clinical laboratory, the clinical picture and serology plays a greater
role in the diagnosis of viral disease. The major types of antibodies that
are assayed for are neutralizing, hemagglutination inhibiting and
complement fixing antibodies. Complement fixing antibodies follow the
kinetics of IgM and are most useful in indicating a current or recent
infection. In contrast the neutralizing and hemagglutinating antibodies
follow the kinetics of IgG, persist for a long time and are used to assess
immunity. The development of antibodies to different components of the
virus is used in staging the disease. For example in hepatitis B and HIV
infections this approach is used.
|
| |
CELLULAR COMPONENTS
In addition to the barriers and
humoral components involved in resistance to and recovery from viral infections,
there are several different cells that play a role in our antiviral defenses.
Nonspecific
Macrophages
By
virtue of the location at various sites in the body, macrophages are one
of the first cells to encounter viruses. Experimental evidence suggest
that these cells play an important role in resistance to viral infection.
For example, newborn mice are susceptible to infection with herpes virus
type 1 due to a defect in the ability of macrophages to prevent
replication of the virus. Macrophages from adult mice however, are able to
prevent replication of the virus and these mice are resistant to infection
with this virus. Also, animals in which macrophages have been depleted are
more susceptible to infection with a variety of viruses. Macrophages
contribute to antiviral defenses in a number of ways.
-
Intrinsic
antiviral activity - Macrophages can be infected with viruses
but many viruses are incapable of replicating in macrophages.
Macrophages that are activated (e.g. by IFN-γ) are even more
capable of resisting viral replication. Thus, macrophages help limit
viral infections by virtue of their intrinsic ability to prevent
replication of viruses. However some viruses are able to replicate or at
least survive in macrophages and thus can be spread by macrophages (see
above).
-
Extrinsic
antiviral activity - Macrophages are also able to recognize
virus infected cells and to kill them. Thus, macrophages also contribute
to antiviral defenses by virtue of their cytotoxic activity.
-
ADCC -
Virus infected cells that are coated with IgG antibodies can be killed
by macrophages by ADCC
-
IFN production
- Macrophages are a source of IFN.
NK Cells
Experimental evidence also suggests that NK cells also play a role in
resistance to viral infection. Mice that are depleted of NK cells are more
susceptible to infection with certain viruses. Also, patients with low NK
cell activity are more susceptible to reoccurrences with herpes simplex
type 1 virus. NK cells act by recognizing and killing virus infected
cells. The recognition of virus infected cells is not MHC-restricted or
antigen specific. Thus, NK cells will kill cells infected with many
different viruses. NK cells can also mediate ADCC and can kill virus
infected cells by this mechanism. The activities of NK cells can be
enhanced by IFN-γ and Il-2 (see above).
|
| |
Specific
T Cells
T cells
play a major role in recovery from viral infections. Cytotoxic T cells (CTLs)
generated in response to viral antigens on infected cells can kill the
infected cells thereby preventing the spread of infection. Helper T cells
are involved in generation of CTLs and in assisting B cells to make
antibody. In addition, lymphokines secreted by T cells can recruit and
activate macrophages and NK cells thereby mobilizing a concerted attack in
the virus.
|
| |
SUMMARY OF DEFENSES
Table 7
(Adapted from: Baron, Medical Microbiology, 2nd Ed., Table 69-2)
summarizes the host defenses against viral infections and it indicates the
targets for each of these defenses.
|
Table 7
Host Effector Functions in
Viral Infections |
|
Host Defense |
Effector |
Target of Effector |
|
Early nonspecific responses |
Fever
Phagocytosis
Inflammation
NK cell activity
Interferon |
Virus replication
Virus
Virus replication
Virus-infected cell
Virus replication,
immunomodulation |
|
Immune responses mediated by
cells |
Cytotoxic T lymphocytes
Activated macrophages
Lymphokines
ADCC |
Virus infected cell
Virus, virus-infected cell
Virus-infected cells,
immunomodulation
Virus-infected cell |
|
Humoral immune responses |
Antibody
Antibody + complement |
Virus, Virus-infected cell
Virus, virus-infected cell |
RELATIVE CONTRIBUTIONS OF HOST
DEFENSE MECHANISMS
The relative contribution of the
various host defense mechanisms will depend on the nature of the virus and the
portal of entry. Antibodies will be more important in infections in which
viremia is a prominent feature. However, antibodies may not be helpful in
infections with herpes or paramyxoviruses in which the virus can be passed
from cell to cell by cell fusion. In this instance cell mediated immunity is
more important. If a virus only infects cells in the mucosal surface, IgA
antibodies may be important.
An understanding of the host
defense mechanisms is important for vaccine development and for proper
administration of vaccines. If IgA antibodies are important for protection
against a particular virus, then any vaccine must be able to stimulate
production of IgA antibodies in the appropriate mucosal surface. Alternatively
if CTLs are important then the vaccine must be able to stimulate CTL
production. That is why live vaccines are often preferable to a killed vaccine
because live vaccines usually lead to the generation of CTLs while killed
vaccines do not.
|
| |
VIRUS-INDUCED IMMUNOPATHOLOGY
Although the host has a variety
of defenses to protect against viral infections, sometimes it is the immune
response to the infection that is the direct cause of tissue injury. For
example, infants infected with cytomegalovirus have circulating immune
complexes that are deposited in the kidneys and joints resulting in pathology
such as arthritis and glomerular nephritis. Another example is fatal
hemorrhagic shock syndrome associated with dengue virus infection. In this
instance fixation of complement by circulating immune complexes results in
release of products of the complement cascade leading to sudden increased
vascular permeability, shock and death.
IMMUNOSUPPRESSION
Many virus are able to suppress
immune responses and thereby overcome or minimize host defenses. The best
example is HIV which infects the CD4+ cells thereby destroying the
specific immune system. Other viruses ( e.g. measles virus) can also
infect lymphocytes and affect their replication and differentiation.
Virus-induced immunosuppression is major concern in vaccine development. Some
of the mechanisms by which viruses can evade host defenses are illustrated in Table
8 (Adapted from: Roitt, Immunology 5th Ed., Fig 16.10).
|
Table 8
Viral Products that
Interfere with Host Defenses |
|
Host Defense Affected |
Virus |
Virus Product |
Mechanism |
|
Interferon |
EBV |
EBERS (small RNAs) |
Blocks protein kinase
activation |
|
Vaccinia |
eIF-2alpha homolog |
Prevents
phosphorylation of eIF-2alpha by protein kinase |
|
Complement |
Vaccinia |
Homologues of
complement control proteins |
Blocks complement
activation |
|
Antibody |
HSV-1 |
gE/gI |
Binds Fc-gamma and
blocks function |
|
Cytokines |
Myxoma |
IFN-gamma receptor
homolog |
Competes for IFN-gamma
and blocks function |
|
Shope fibroma virus |
TNF receptor |
Competes for TNF and
blocks function |
|
EBV |
IL-10 homolog |
Reduces IFN-gamma
function |
|
MHC Class I |
CMV |
Early protein |
Prevents transport of
peptide-loaded MHC |
|
Adenovirus |
E3 |
Blocks transport of MHC
to surface |
|
Apoptosis |
Adenovirus |
14.7K |
Inhibits capsases |
|
EBV |
Bcl-2 homolog |
Anti-apoptotic |
|
NK cells |
HCMV |
UL-18 |
MHC homolog inhibits NK
cells |
|
|
|
 Return to the Virology section of Microbiology and Immunology On-line
Return to the Virology section of Microbiology and Immunology On-line
 Return to the Home Page of Microbiology and Immunology On-line
Return to the Home Page of Microbiology and Immunology On-line
This page last changed on
Saturday, October 29, 2016
Page maintained by
Richard Hunt
|

 Fig. 1. The discovery of interferon
Fig. 1. The discovery of interferon
 Fig. 3. Mode of action of interferon
Fig. 3. Mode of action of interferon Fig. 4 Molecular basis of the antiviral state
Fig. 4 Molecular basis of the antiviral state Fig. 5 Effects of interferons alpha, beta and gamma
Fig. 5 Effects of interferons alpha, beta and gamma
